Validation of Superiority of p40 over p63 in Differentiating Squamous Cell Carcinoma and Adenocarcinoma Lung
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(04): 535-542
DOI: DOI: 10.4103/ijmpo.ijmpo_51_19
Abstract
Context
In this era of targeted therapy, it is important to distinguish the various subtypes of nonsmall cell lung carcinoma (NSCC). Diagnosis based on morphology alone is challenging in poorly differentiated carcinomas and core biopsies. Immunohistochemistry (IHC) helps in specifying the lineage for the subtype of NSCC. Till date, p63 is the most frequently used and sensitive marker for squamous cell carcinoma (SQCC). However, it is not specific and stains a subset of adenocarcinoma (ADC). Thus, a more reliable and specific marker is required for the diagnosis of SQCC.
Objective
The objective of the study was to validate the diagnostic utility of p40 over p63 in differentiating pulmonary SQCC from ADC and NSCC-not otherwise specified (NOS).
Materials and Methods
A total of 123 cases of NSCC were initially reviewed and subtyped blinded to the results of IHC. This was followed by a review of IHC slides which included p63, p40, thyroid transcription factor 1, Napsin-A, cytokeratin (CK) 5/6, and CK7.
Results
There were 64 ADC, 19 SQCC, and 40 NSCC-NOS. IHC helped to confirm the morphological diagnosis in 62/64 ADCs and19/19 SQCCs. IHC classified the cases of NSCC-NOS into NSCC favoring ADC – 12 cases, NSCC favoring SQCC – 10 cases, and NSCC favoring AD-SQCC – 4 cases. Both p63 and p40 showed near equal sensitivity for SQCC (100% and 97%, respectively), whereas p63 showed far lower specificity when compared to p40 (51.3% vs. 100%).
Conclusion
The present study confirms and validates that p40 is equally sensitive but highly specific than p63 in detecting SQCC. Hence, we recommend the routine use of p40 instead of p63 for the definite categorization of NSCC of the lung.
Publication History
Received: 26 February 2019
Accepted: 29 June 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context
In this era of targeted therapy, it is important to distinguish the various subtypes of nonsmall cell lung carcinoma (NSCC). Diagnosis based on morphology alone is challenging in poorly differentiated carcinomas and core biopsies. Immunohistochemistry (IHC) helps in specifying the lineage for the subtype of NSCC. Till date, p63 is the most frequently used and sensitive marker for squamous cell carcinoma (SQCC). However, it is not specific and stains a subset of adenocarcinoma (ADC). Thus, a more reliable and specific marker is required for the diagnosis of SQCC.
Objective
The objective of the study was to validate the diagnostic utility of p40 over p63 in differentiating pulmonary SQCC from ADC and NSCC-not otherwise specified (NOS).
Materials and Methods
A total of 123 cases of NSCC were initially reviewed and subtyped blinded to the results of IHC. This was followed by a review of IHC slides which included p63, p40, thyroid transcription factor 1, Napsin-A, cytokeratin (CK) 5/6, and CK7.
Results
There were 64 ADC, 19 SQCC, and 40 NSCC-NOS. IHC helped to confirm the morphological diagnosis in 62/64 ADCs and19/19 SQCCs. IHC classified the cases of NSCC-NOS into NSCC favoring ADC – 12 cases, NSCC favoring SQCC – 10 cases, and NSCC favoring AD-SQCC – 4 cases. Both p63 and p40 showed near equal sensitivity for SQCC (100% and 97%, respectively), whereas p63 showed far lower specificity when compared to p40 (51.3% vs. 100%).
Conclusion
The present study confirms and validates that p40 is equally sensitive but highly specific than p63 in detecting SQCC. Hence, we recommend the routine use of p40 instead of p63 for the definite categorization of NSCC of the lung.
Introduction
In the past, the only important distinction necessary was between small cell lung carcinoma (SCLC) and non-SCLC (NSCC). As targeted therapies have evolved for various subtypes of NSCC, there is an ever-increasing need for differentiating these subtypes. Distinguishing adenocarcinoma (ADC) and squamous cell carcinoma (SQCC) is important as the treatment protocols differ.
To a large extent, the hematoxylin and eosin (H and E)-stained sections are enough to study the morphology for a definite diagnosis. However, in poorly differentiated carcinomas, and more importantly for small/core biopsies, difficulty arises in subtyping NSCC accurately. It is mandatory for the small/core biopsies to be preserved for detecting the driver mutations making the tissue available for any required ancillary tests, especially immunohistochemistry (IHC) even less.
There are various IHC markers available to subtype NSCC. For ADC, the more important ones are the nuclear marker TTF-1 and the cytoplasmic marker, Napsin-A. SQCC has sensitive antibody markers like the nuclear marker, p63, and the cytoplasmic and membrane staining cytokeratin 5/6 (CK 5/6) antibody, among others. Novel antibodies always take antibody detection to new levels by being more specific and/or sensitive.
Till date for SQCC, anti-p63 antibody is the most frequently used nuclear marker. Although it has good sensitivity, it is not highly specific as it also stains other NSCCs. It is not useful, especially in cases of poorly differentiated NSCC-not otherwise specified (NOS) as it stains 15%–65%-of ADC.[1] Hence, there is a need for a reliable, easy to assess, and a more specific marker in diagnosing SQCC.
The anti-p40 antibody is highly specific for squamous/basal cells. Studies have shown this marker to have similar sensitivity as p63 and markedly high specificity for SQCC, as it stains a lesser number of ADC. The potential pitfall of diagnosing a p63-positive ADC as SQCC can be averted to a large extent if p40 is used. In this study, we have validated the utility of p40 over p63 in differentiating pulmonary SQCC from ADC and NSCC-NOS in a large cohort of biopsies and resected specimens (RSs).
Materials and Methods
A total number of 123 consecutive cases of NSCC diagnosed on core-needle biopsies (CNBs), endobronchial biopsies (EBBs), and RSs of the lung sent for histopathological evaluation to the department of pathology between December 2014 and May 2016 were analyzed prospectively. This study was approved by the Institutional Ethics Committee. The demographic data, clinical details, radiological features, and laboratory investigations were retrieved from the medical records. The SCLCs, carcinoids, lymphomas, mesenchymal neoplasms, metastatic carcinomas, and mesotheliomas were excluded from the study.
Morphological analysis
Initially, all the cases of NSCC were morphologically categorized based on the review of H and E slides blinded to the results of special stains and IHC results. Subsequently, special stains with Alcian–periodic acid–Schiff for mucin were reviewed wherever performed.
Immunohistochemistry
In all cases where IHC was done, the slides were reviewed to subtype the NSCC. In those cases where IHC was not done, the initial morphological diagnosis was considered as a final diagnosis. The primary panel of SQCC and ADC markers included p40, p63, CK5/6, TTF1, Napsin-A, and CK7. All IHCs were performed on fully automated immunostainer (Xmatrx Elite; BioGenex) by Poly horse raddish peroxide (Poly HRP) technique. Of the primary antibodies used, Napsin-A was supplied by Biocare, p40 by Pathinsitu, and the rest by BioGenex. All were monoclonal antibodies except p40.
All cases of NSCC were categorized as per the proposed International Association for the Study of Lung Cancer American Thoracic Society European Respiratory Society classification for small biopsies.[2],[3] For all the markers, the intensity of staining was taken into consideration and was compared to positive controls. For the SQCC markers, p40 and p63, nuclear staining was accepted, and cytoplasmic staining was ignored. For p40 and p63 antibody, the intensity of staining was scored semi-quantitatively using a 3-tier system: weakly positive (Grade 1), moderate positivity (Grade 2), and strongly positive (Grade 3). The staining proportion pattern was scored on a 4-tier system: <5>50%.-Cases showing positivity of 5%-or less were considered negative. However, H-score was not determined.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of both the ADC and SQCC markers were analyzed.
Results
Of the 123 patients, nearly three-fourth, 92/123 (74.8%) were male with a male-to-female ratio of 3:1. The age of the patients ranged from 18 years to 84 years, with a mean age of 57.8 years among males and 51.8 among females. Nearly 94.3%-of the samples received were small biopsies, with only a small fraction of 7 (5.6%) cases being surgical resection specimen. Of the biopsies, majority were computed tomography-guided CNB [95/123 (77.2%)], followed by EBB [20/123 (16.2%)]. There was only one biopsy which was done under ultrasound guidance.
Histopathologic examination
ADC was the most common histological subtype accounting for more than 60%-(74/123) of NSCC cases. Of these, more than 80%-(62/74) were diagnosed on H and E slides alone with the remaining 12 cases (16.2%)-requiring IHC for subcategorization. Of the total 64 ADC cases diagnosed on H and E morphology, majority (46 [71.9%]), were acinar type, followed by lepidic type (10 [15.6%]), mucinous (4 [6.3%]), papillary (3 [4.7%]), and fetal subtype 1 (1.5%). Most of the acinar-type ADCs showed pure acinar morphology (38 cases) with remaining showing predominantly acinar morphology in combination with other morphologies like solid pattern (4 cases) and 1 case each of papillary, cribriform, and focal micropapillary pattern. There was one case which had an additional combination of lepidic as well as micropapillary pattern. Two of the four cases which showed predominant acinar morphology in combination with solid areas were categorized as adenosquamous carcinoma (AD-SQCC) after IHC. These two cases showed expression of SQCC markers in solid areas and ADC markers in acinar areas. Of the 10 cases of lepidic subtype, 8 cases had a pure lepidic pattern and 2 cases had additional micropapillary and acinar patterns.
Although NSCC-NOS formed the second major subtype, 40/123 (32.5%)-on H and E morphology, 24 (60%)-of these were further subtyped on IHC. These included 12 cases as NSCC favoring ADC, 10 cases favoring SQCC, and the remaining 2 cases as favoring AD-SQCC. Still, 16/123 (13%)-cases could not be further subcategorized and were retained as NSCC-NOS. Of these 16 cases, IHC slides were not available in 6 cases and the rest 10 remained uncategorized even after an appropriate panel of IHC.
SQCCs accounted for 23.6% (29/123) of the cases of NSCC in the present study. Of these, 19 (65.5%) were diagnosed on H and E morphology alone, while the remaining (10 [34.5%]) required IHC to favor a diagnosis of SQCC. The distribution of the cases based on histological subtype before and after IHC is provided in [Table 1].
|
H and E diagnosis (before IHC) |
n |
Final diagnosis (after IHC*) |
n |
|---|---|---|---|
|
*IHC was not done in all cases. In such cases where IHC was not done initial morphological diagnosis was considered as final diagnosis. H and E – Hematoxylin and Eosin; IHC – Immunohistochemistry; ADC – Adenocarcinoma; SQCC – Squamous cell carcinoma; NSCC – Nonsmall cell carcinoma; NOS – Not otherwise specified; AD-SQCC – Adenosquamous carcinoma |
|||
|
ADC |
64 |
ADC |
62 |
|
NSCC favor AD-SQCC |
2 |
||
|
SQCC |
19 |
SQCC |
19 |
|
NSCC-NOS |
40 |
NSCC-NOS |
16 |
|
NSCC favor ADC |
12 |
||
|
NSCC favor SQCC |
10 |
||
|
NSCC favor AD-SQCC |
2 |
||
|
Total |
123 |
Total |
123 |
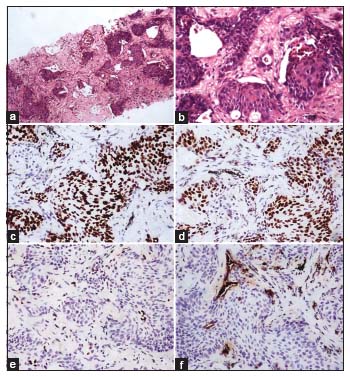
| Figure 1:(a and b) Squamous cell carcinoma (H and E; a, ×40; b, ×100). (c) p63 and (d) p40 showing diffuse strong nuclear staining in the tumor cells (c and d, ×100). (e) TTF‑1, negative in tumor cells. Few cells showing nuclear staining at the periphery of tumor nests are normal alveolar epithelial cells which serve as internal positive control (e, ×100). (f) Napsin A, negative in tumor cells. Few cells showing granular cytoplasmic staining at the periphery of tumor nests are normal alveolar epithelial cells which serve as internal positive control (f, ×100)
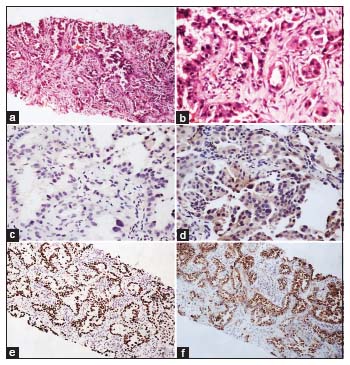
| Figure.2:(a and b) Adenocarcinoma showing predominantly lepidic pattern with focal acinar pattern (H and E; a, ×40; b, ×100). Both (c) p63 and (d) p40 being negative in the tumor cells. Please note nonspecific cytoplasmic staining with p40 (c and d, ×100). (e) TTF‑1 showing diffuse strong nuclear staining in tumor cells (e, ×40). (f) Napsin A showing diffuse strong granular cytoplasmic staining in tumor cells (f, ×40)
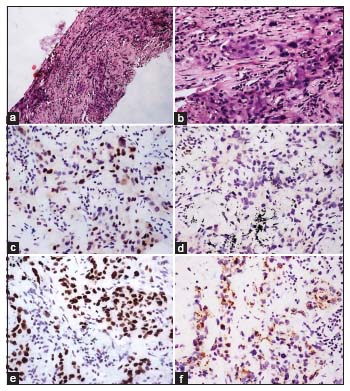
| Figure.3:(a and b) Adenocarcinoma showing lepidic, acinar, and focal micropapillary patterns (H and E; a, ×40; B, ×100). (c and d) p63 showing nuclear positivity in the tumor cells. Staining of nuclei of the basal cells of bronchiolar epithelium serves as internal positive control (c, ×40; d, ×100). (e and f) p40 being negative in tumor cells. Staining of nuclei of the basal cells of bronchiolar epithelium serves as internal positive control (e, ×40; f, ×100)
Squamous cell carcinoma markers
As stated earlier, SQCC markers, p63 and p40, which were the main subjects of the present study, were performed in 107/123 (86.9%) NSCC cases, which included both morphologically differentiated and undifferentiated NSCC cases. Both p63 and p40 showed near equal sensitivity for SQCC (100% and 97%, respectively), whereas p63 showed far lower specificity when compared to p40 (51.3% vs. 100%). Only one case in which p40 was negative but p63 was positive was a case of NSCC-NOS on initial morphology which was later categorized as NSCC favoring SQCC after IHC. This case showed additional positivity for CK5/6 and was negative for both TTF-1 and Napsin-A. None of the ADC cases were positive for p40, whereas p63 was positive in half of the cases of ADC including majority of the morphologically well-differentiated ones. Majority of ADC showed Grade 1 to Grade 2 positivity in 5%–50%-of cells. However, there were also cases showing Grade 3 staining, as well as staining in more than 50%-cells. There were five cases where p63 showed weak staining in 5%–25%-which were not categorized as NSCC favoring SQCC and retained as NSCC-NOS. These five cases were negative for p40 and two of these where CK5/6 was also done showed negative staining. In one of these cases, ADC markers were negative, while in one case, they were not performed.
Of the total 4 cases finally categorized as NSCC favoring AD-SQCC, two were morphologically ADC (predominantly acinar with focal solid areas) and the remaining two were morphologically undifferentiated (NSCC-NOS). In all these cases, there were distinct areas of staining for ADC markers (TTF-1 and Napsin-A) and SQCC markers p40 and CK5/6 (done in 1 case), but the marker p63 showed overlapping positivity both in squamous as well as in glandular areas.
Adenocarcinoma markers
This study also documented high sensitivities of the markers TTF-1 and Napsin-A for ADC. The sensitivity of TTF-1 and Napsin-A was 92.42%-and 89.23%, respectively. Although TTF-1 showed slightly higher sensitivity compared to Napsin-A, both were 100%-specific. One case of invasive mucinous ADC showed negativity for both TTF-1 and Napsin-A, while for another double-negative case, no primary was found in any other part of the body even after extensive investigations; hence, it was categorized as primary lung ADC. This case was a morphologically well-differentiated ADC of acinar type and was positive for CK7.
There were four cases of ADC which showed discordant staining for Napsin-A and TTF-1. Two of cases were Napsin-A postive and TTF-1 negative, with other two showing the reverse pattern of staining. None of the ADC markers were positive in SQCC. As stated earlier, both TTF-1 and Napsin-A distinctly highlighted the ADC areas, while the remaining negative in SQCC areas of all four cases of NSCC favor AD-SQCC. [Figure 4] and [Figure 5] show cases categorized as NSCC favoring ADC and SQCCC after IHC, respectively. [Figure 6] depicts adenosquamous carcinoma which was initially categorized as ADC with solid and acinar patterns on morphology.
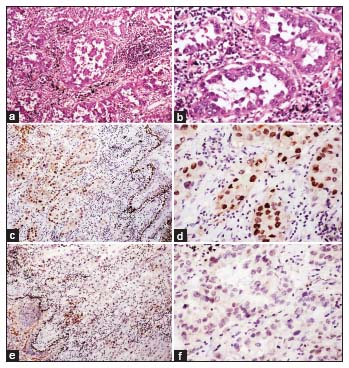
| Figure.4:(a and b) Nonsmall cell lung carcinoma‑not otherwise specified on morphology showing nests and cords of tumor cells infiltrating the fibrous stroma (H and E; a, ×40; b, ×100). (c) p63 showing positivity within the tumor cells (×100). (d) p40 being negative in tumor cells. (e) TTF‑1 showing diffuse strong nuclear staining in tumor cells (e × 100). (f) Napsin‑A showing moderate granular cytoplasmic staining in tumor cells. This case was later categorized as nonsmall cell lung carcinoma favour adenocarcinoma after immunohistochemistry

| Figure.5:(a and b) Nonsmall cell lung carcinoma‑not otherwise specified on morphology showing nests and cords of tumor cells infiltrating the fibrous stroma (H and E; a, ×40; b, ×100). (c) p63 and (d) p40 showing positivity within the tumor cells (c, ×100; d, ×100). (d) TTF‑1 and (e) Napsin‑A being negative in tumor cells (e, ×100; f, ×100). This case was later categorized as nonsmall cell lung carcinoma favoring SQCCC after immunohistochemistry

| Figure.6:(a and b) Adenosquamous carcinoma which was initially categorized as adenocarcinoma with solid and acinar pattern on morphology (H and E; a, ×40; b, ×100). (c) p63 Showing diffuse nuclear staining in solid areas. Few nuclei in the glandular areas also show positive staining. (c, ×100). (d) p40 also showed diffuse nuclear staining in solid areas but negative in glandular areas (d, ×100). (e) TTF‑1 showing positive nuclear staining in glandular areas but negative in (f) solid areas (e, ×100; f, ×100). (g) Napsin‑A showing moderate granular cytoplasmic staining in tumor cells but is negative in (h) solid areas (g, ×100; h, ×100)
The sensitivity, specificity, PPV, and NPV of ADC and SQCC markers are provided in [Table 4] and [Table 5], respectively.
|
IHC marker |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
||||
|---|---|---|---|---|---|---|---|---|
|
Value |
95% CI |
Value |
95% CI |
Value |
95% CI |
Value |
95% CI |
|
|
IHC – Immunohistochemistry; CI – Confidence interval; PPV – Positive predictive value; NPV – Negative predictive value |
||||||||
|
P63 |
100.00 |
89.42-100.00 |
51.25 |
39.81-62.59 |
45.83 |
34.02-58.00 |
100.00 |
91.4-100.00 |
|
P40 |
96.97 |
84.24-99.92 |
100.00 |
94.72-100.00 |
100.00 |
89.11-100.00 |
98.55 |
92.19-99.96 |
|
CK5/6 |
72.73 |
39.03-93.98 |
60.87 |
38.54-80.29 |
47.06 |
22.98-72.19 |
82.35 |
56.57-96.20 |
|
Study |
p-40 |
p63 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Type of antibody |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Type of antibody |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
|
|
PPV – Positive predictive value; NPV – Negative predictive value |
||||||||||
|
Bishop et al., 2012 (n=470)[1] |
Polyclonal |
100 |
98 |
92 |
100 |
Monoclonal |
100 |
64 |
34 |
100 |
|
Righi et al., 2011 (n=103)[11] |
- |
100 |
96 |
94 |
100 |
Monoclonal |
96 |
80 |
64 |
98 |
|
Tacha et al., 2013 (n=90)[12] |
Monoclonal |
85 |
98 |
- |
- |
Monoclonal |
85 |
90 |
- |
- |
|
Polyclonal |
85 |
98 |
- |
- |
||||||
|
Collins et al., 2013 (n=100)[13] |
Monoclonal |
89.4 |
100 |
100 |
93.9 |
Monoclonal |
86.8 |
96.7 |
94.3 |
92.3 |
|
Ming-Hui et al., 2014 (n=200)[8] |
- |
80.5 |
90 |
- |
- |
Monoclonal |
93.5 |
80 |
- |
- |
|
Dvorak et al., 2016 (n=538)[14] |
Monoclonal (BC28) |
92.6 |
94.8 |
94.1 |
93.8 |
Monoclonal |
92.1 |
89.2 |
88.3 |
92.6 |
|
Monoclonal (VP Echelon 40) |
75 |
98.6 |
97.9 |
81.6 |
||||||
|
Present study |
Polyclonal |
96.97 |
100 |
100 |
98.55 |
Monoclonal |
100 |
51.25 |
45.83 |
100 |

| Figure 1:(a and b) Squamous cell carcinoma (H and E; a, ×40; b, ×100). (c) p63 and (d) p40 showing diffuse strong nuclear staining in the tumor cells (c and d, ×100). (e) TTF‑1, negative in tumor cells. Few cells showing nuclear staining at the periphery of tumor nests are normal alveolar epithelial cells which serve as internal positive control (e, ×100). (f) Napsin A, negative in tumor cells. Few cells showing granular cytoplasmic staining at the periphery of tumor nests are normal alveolar epithelial cells which serve as internal positive control (f, ×100)

| Figure.2:(a and b) Adenocarcinoma showing predominantly lepidic pattern with focal acinar pattern (H and E; a, ×40; b, ×100). Both (c) p63 and (d) p40 being negative in the tumor cells. Please note nonspecific cytoplasmic staining with p40 (c and d, ×100). (e) TTF‑1 showing diffuse strong nuclear staining in tumor cells (e, ×40). (f) Napsin A showing diffuse strong granular cytoplasmic staining in tumor cells (f, ×40)

| Figure.3:(a and b) Adenocarcinoma showing lepidic, acinar, and focal micropapillary patterns (H and E; a, ×40; B, ×100). (c and d) p63 showing nuclear positivity in the tumor cells. Staining of nuclei of the basal cells of bronchiolar epithelium serves as internal positive control (c, ×40; d, ×100). (e and f) p40 being negative in tumor cells. Staining of nuclei of the basal cells of bronchiolar epithelium serves as internal positive control (e, ×40; f, ×100)

| Figure.4:(a and b) Nonsmall cell lung carcinoma‑not otherwise specified on morphology showing nests and cords of tumor cells infiltrating the fibrous stroma (H and E; a, ×40; b, ×100). (c) p63 showing positivity within the tumor cells (×100). (d) p40 being negative in tumor cells. (e) TTF‑1 showing diffuse strong nuclear staining in tumor cells (e × 100). (f) Napsin‑A showing moderate granular cytoplasmic staining in tumor cells. This case was later categorized as nonsmall cell lung carcinoma favour adenocarcinoma after immunohistochemistry

| Figure.5:(a and b) Nonsmall cell lung carcinoma‑not otherwise specified on morphology showing nests and cords of tumor cells infiltrating the fibrous stroma (H and E; a, ×40; b, ×100). (c) p63 and (d) p40 showing positivity within the tumor cells (c, ×100; d, ×100). (d) TTF‑1 and (e) Napsin‑A being negative in tumor cells (e, ×100; f, ×100). This case was later categorized as nonsmall cell lung carcinoma favoring SQCCC after immunohistochemistry

| Figure.6:(a and b) Adenosquamous carcinoma which was initially categorized as adenocarcinoma with solid and acinar pattern on morphology (H and E; a, ×40; b, ×100). (c) p63 Showing diffuse nuclear staining in solid areas. Few nuclei in the glandular areas also show positive staining. (c, ×100). (d) p40 also showed diffuse nuclear staining in solid areas but negative in glandular areas (d, ×100). (e) TTF‑1 showing positive nuclear staining in glandular areas but negative in (f) solid areas (e, ×100; f, ×100). (g) Napsin‑A showing moderate granular cytoplasmic staining in tumor cells but is negative in (h) solid areas (g, ×100; h, ×100)
References
- Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol 2012; 25: 405-15
- Thunnissen E, Kerr KM, Herth F. The challenge of NSCC diagnosis and predictive analysis on small samples. Practical approach of a working group. LungCancer 2012; 76: 1-188
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y. et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244-85
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10-29
- Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCC subtypes and their therapeutic implications. Lung Cancer 2013; 82: 179-89
- Mitsudomi T, Morita S, Yatabe Y. Gefitinib versus cisplatin plus docetaxel in patients with nonsmall cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121-28
- Travis WD, Rekhtman N, Riley GJ, Geisinger KR, Asamura H, Brambilla E. et al. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: A paradigm shift. J Thorac Oncol 2010; 5: 411-4
- AO MH, Zhang H, Sakowski L, Sharma R, Illei PB, Gabrielson E. et al. The utility of a novel triple marker (combination of TTF1, napsin A, and p40) in the subclassification of non-small cell lung cancer. Hum Pathol 2014; 45: 926-34
- Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction-a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol 2006; 3: 448-57
- ;Nonaka D. A study of ΔNp63 expression in lung non-small cell carcinomas. Am J Surg Pathol 2012; 36: 895-9
- ;Righi L, Graziano P, Fornari A, Rossi G, Barbareschi M, Cavazza A. et al. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology a retrospective study of 103 cases with surgical correlation. Cancer 2011; 117: 3416-23
- ;Tacha D, Bremer R, Haas T, Qi W. An immunohistochemical analysis of a newly developed, mouse monoclonal p40 (BC28) antibody in lung, bladder, skin, breast, prostate, and head and neck cancers. Arch Pathol Lab Med 2014; 138: 1358-64
- Collins BT, Wang JF, Bernadt CT. Utilization of p40 (ΔNp63) with p63 and cytokeratin5/6 immunohistochemistry in non-small cell lung carcinoma fine-needle aspiration biopsy. Acta Cytologica 2013; 57: 619-24
- Dvorak K, Yambert J, Palting J, Reinhardt K, Roessler C, Moh A. et al. Improved lung cancer classification using new optimized immunohistochemical assay with anti-p40 (BC28) mouse monoclonal antibody. Int J Clin Exp Pathol 2016; 9: 2693-701


 PDF
PDF  Views
Views  Share
Share

