Uterine carcinosarcomas: 8-year single center experience of 25 cases
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(03): 149-153
DOI: DOI: 10.4103/0971-5851.92814
Abstract
Objectives: The aim of this retrospective study was to evaluate the behavior and treatment outcomes of uterine carcinosarcomas in relation to their clinical and pathogenic features and to determine the optimal treatment strategy. Secondary objectives were to identify parameters predictive of survival. Materials and Methods: The hospital records of all 25 patients of uterine carcinosarcoma operated between 2000 and 2008 in Gujarat cancer research institute, Ahmedabad, were reviewed. Patients who presented with clinical evidence of recurrent disease or those who had incomplete medical records were excluded from our analysis. The status of these patients was updated up to November, 2010. Patients were classified according to the new 2009 FIGO staging system for endometrial carcinoma, to see what difference the assigned stage has on survival with the old treatment strategy. Survival was calculated by Kaplan-Meier method and compared by Log-Rank test. Median survival time was derived with the Brookmeyer 95% confidence interval. For comparison of qualitative data, Chi-Square test and Fisher extract c2 were used. Results: Median age of patients was 56 years (range, 36-77 years). Only 36% of patients had stage I at diagnosis and another 36% were stage III. Most of the tumors (56%) were with homologous sarcomatous components and 64% of tumors were high grade (grade 2/3) at diagnosis. Fifty-two percent patients received postoperative adjuvant treatment. Twelve patients had no postoperative treatment: two were lost to follow-up immediately after surgery, four could not receive adjuvant treatment on account of severe medical complications and age factor which could have increased morbidity, and six patients declined treatment. Four of these patients expired within one year of diagnosis, two other within 18 months, and rest were lost to follow-up. The difference in survival of 13 patients who had taken adjuvant treatment was significantly more than the group who had not taken adjuvant therapy (P=0.025). The overall 3-year disease-free survival of 13 patients who had taken adjuvant therapy was 40%. However, these adjuvant treatment modalities had borderline statistical significance on overall survival of patients (P=0.075). The only statistically significant predictor of survival in this study was stage of the disease (P=0.035). Conclusions: This highly aggressive uterine malignancy warrants comprehensive surgical staging to assess tumor dissemination followed by systematic adjuvant therapy in patients with both early and advanced disease. The value of pelvic Radiotherapy in addition to systemic treatment remains ill-defined. Stage is the significant predictor of survival for the disease. Our results indicate that in this highly aggressive malignancy, further exploration of potential outcome benefits of postoperative treatment, especially chemoradiation, is warranted in larger group of patients after comprehensive surgical staging.
Publication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objectives:
The aim of this retrospective study was to evaluate the behavior and treatment outcomes of uterine carcinosarcomas in relation to their clinical and pathogenic features and to determine the optimal treatment strategy. Secondary objectives were to identify parameters predictive of survival.
Materials and Methods:
The hospital records of all 25 patients of uterine carcinosarcoma operated between 2000 and 2008 in Gujarat cancer research institute, Ahmedabad, were reviewed. Patients who presented with clinical evidence of recurrent disease or those who had incomplete medical records were excluded from our analysis. The status of these patients was updated up to November, 2010. Patients were classified according to the new 2009 FIGO staging system for endometrial carcinoma, to see what difference the assigned stage has on survival with the old treatment strategy. Survival was calculated by Kaplan-Meier method and compared by Log-Rank test. Median survival time was derived with the Brookmeyer 95% confidence interval. For comparison of qualitative data, Chi-Square test and Fisher extract χ2 were used.
Results:
Median age of patients was 56 years (range, 36-77 years). Only 36% of patients had stage I at diagnosis and another 36% were stage III. Most of the tumors (56%) were with homologous sarcomatous components and 64% of tumors were high grade (grade 2/3) at diagnosis. Fifty-two percent patients received postoperative adjuvant treatment. Twelve patients had no postoperative treatment: two were lost to follow-up immediately after surgery, four could not receive adjuvant treatment on account of severe medical complications and age factor which could have increased morbidity, and six patients declined treatment. Four of these patients expired within one year of diagnosis, two other within 18 months, and rest were lost to follow-up. The difference in survival of 13 patients who had taken adjuvant treatment was significantly more than the group who had not taken adjuvant therapy (P=0.025). The overall 3-year disease-free survival of 13 patients who had taken adjuvant therapy was 40%. However, these adjuvant treatment modalities had borderline statistical significance on overall survival of patients (P=0.075). The only statistically significant predictor of survival in this study was stage of the disease (P=0.035).
Conclusions:
This highly aggressive uterine malignancy warrants comprehensive surgical staging to assess tumor dissemination followed by systematic adjuvant therapy in patients with both early and advanced disease. The value of pelvic Radiotherapy in addition to systemic treatment remains ill-defined. Stage is the significant predictor of survival for the disease. Our results indicate that in this highly aggressive malignancy, further exploration of potential outcome benefits of postoperative treatment, especially chemoradiation, is warranted in larger group of patients after comprehensive surgical staging.
INTRODUCTION
Carcinosarcoma of uterus, also known as malignant mixed mullerian tumor (MMMT) of uterus, in contrast to endometrial carcinoma is very rare but highly aggressive neoplasm that usually arises in postmenopausal age group. It represents the most frequent uterine sarcoma, 1.5% of all malignant uterine cancers.[1] The frankly malignant variants grow rapidly and usually are accompanied by postmenopausal bleeding, pelvic pain, a palpable lower abdominal mass, or symptoms of metastatic disease. Most patients have enlarged uterus and the tumor protrudes through cervical os in half the patients.[2] It accounts for 16.4% of deaths attributable to uterine malignancies. Equally disconcerting is the lack of measurable improvement in patient's survival over the past four decades, despite more aggressive adjuvant therapeutic strategies.[3,4] Most, but not all, uterine carcinosarcomas are monoclonal tumors and really metaplastic carcinomas. Metastases are usually from carcinomatous element and sarcomatous element is believed to be derived as a result of dedifferentiation of the carcinomatous component.[2]
MMMT of uterus are associated with a less favorable outcome than uterine carcinoma with 5-year survival rates ranging between 33 and 39%.[4] The most important prognostic factor is the stage of disease at the time of treatment. Survival is very poor when tumor extends beyond the uterus. The prognostic impact of other clinical and pathology parameters is controversial.[5]
The primary treatment of MMMT of uterus is surgery but high rates of both local and distant relapse after surgery presupposes the need for effective adjuvant therapies. Most recurrences develop within 12 months and at distant sites.[6] There is neither clear evidence that adjuvant radiotherapy, chemotherapy, or both improve the overall survival of these patients, nor there is clear consensus regarding therapeutic strategies for different stages of disease.[7] The aim of this retrospective study was to evaluate the behavior and treatment outcomes of uterine carcinosarcomas in relation to their clinical and pathogenic features and to determine the optimal treatment strategy. Secondary objectives included identification of parameters predictive of survival. As staging classification by International Federation of Obstetrics and Gynecology (FIGO) has changed in 2009, we applied this change on staging distribution and survival by stage, to see what difference the assigned stage has on survival with the old treatment strategy; however, no new treatment guidelines have been laid down in accordance with changed staging.
MATERIALS AND METHODS
The hospital records of all 25 patients of uterine carcinosarcoma operated during the period 2000-2008 in Gujarat Cancer Research Institute, Ahmedabad, were reviewed. Patients who presented with clinical evidence of recurrent disease or those who had incomplete medical records were excluded from our analysis. Institutional review board approval was obtained for the study performed. Data were retrieved from the patient's records, institutional tumor registry, and death certificates. The diagnosis was based on the original histology report signed out by two certified pathologists. The status of these patients was updated up to November, 2010. These data were used to update the patient's surgical-pathological stage of disease based on new FIGO staging for the endometrial carcinoma, which is also recommended for uterine carcinosarcomas.[8] Information regarding treatment, including surgery, chemotherapy, and/or radiation therapy, and follow up was collected. Patients were considered appropriately surgically staged if a lymphadenectomy was performed in stages I to III. Omentectomy was not considered part of staging procedure. With demonstrable intra-abdominal or distant metastasis, a biopsy of extrapelvic disease was considered sufficient for surgical staging. Patients were considered unstaged when they were operated outside institute and had improper or unavailable documents. Treatment failures detected in central pelvis, pelvic sidewall, pelvic and/or aortic lymph nodes were considered locoregional recurrences. Distant recurrences were defined as disease recurring in upper abdomen or in extra-abdominal sites after initial treatment. Disease-free survival (DFS) or time to recurrence after treatment was defined from date of completion of first treatment to first diagnosis of recurrent disease.
Statistical methods
SPSS (Statistical package for social sciences) for windows 13 program was used for statistical analyses in evaluating findings obtained in the study. In addition to the defining statistical methods (median, standard deviation, frequency), for comparison of qualitative data, Chi-Square test and Fisher exact χ2 were used. Kaplan Meier survival analysis was used for survival analyses and Log Rank test was used for comparing survival data. Median survival time was derived with the Brookmeyer 95% confidence interval.
RESULTS
During the study period, 25 patients with primary uterine carcinosarcoma of uterus met the inclusion criteria. Clinical and therapeutic variables for this cohort of patients are described in Table 1. All patients underwent total abdominal hysterectomy, surgical staging except for two patients who were unstaged. Median age of patients was 58 years (range, 36-77 years). Only 36% of patients had stage I at diagnosis, another 36% were stage III, and 20% were in stage II and IV. Most of the tumors (56%) had homologous sarcomatous components and 72% of tumors were high grade (grade 2/3) at diagnosis. There was no patient with a history of pelvic radiotherapy in past in our series. Only five patients in study were premenopausal, all the rest had reached menopause spontaneously. Two of the premenopausal patients had surgical menopause at the time of presentation: one had undergone Wertheim's hysterectomy with bilateral pelvic lymph node dissection and the other had total abdominal hysterectomy with bilateral salpingo-oophorectomy. All the 20 postmenopausal patients had spontaneous menopause with ten of them operated outside. The presenting symptom was bleeding (majority with postmenopausal bleeding and mennorhagia in premenopausal females) in 20 (80%) patients, pain in six (24%), abdominal mass or swelling in two, and discharge per vagina in two patients. In some patients, there was more than one symptom. One patient was found to have concomitant ovarian malignancy. Table 2 presents the details of symptoms. Mean follow-up period was 16 months (range, 3-65 months). Fifty-two percent patients had received postoperative adjuvant treatment. Twelve of 25 patients had taken no postoperative treatment: 2/12 were lost to follow-up immediately after surgery, 4/12 could not receive adjuvant treatment on account of severe medical complications and age factor which could have increased morbidity, and 6/12 patients declined treatment. Four of these 12 patients expired within one year of diagnosis, two other within 18 months, and rest were lost to follow-up. Four patients were refused adjuvant treatment: First was 60-year-old patient with poor performance status in stage IIIA G3 and died after nine months of surgery after distant and locoregional recurrence due to multiorgan failure; second was 77-year-old female in stage IB G3 with postoperative burst abdomen as complication and died natural death six months after surgery with no sign of recurrence; third was 62-year-old patient in stage IA G1 with histological predominant adenosarcoma, had 11-month disease-free period after which developed unresectable pelvic recurrence, received palliative chemotherapy for it, and died three months after completion of chemotherapy due to cardiac complications; fourth patient was 50-year-old in stage II G2 with associated controlled diabetes mellitus, developed ischemic heart disease on fourth postoperative day, and died after six months of surgery without recurrence due to cardiac complication. Among patients in no adjuvant treatment arm, six patients could not receive postoperative treatment on account of associated medical comorbidity, residual disease, and advanced age with poor performance status. All these factors may have definitely affected the overall survival in no adjuvant treatment arm: four of these six patients expired within one year of diagnosis and two other within 18 months. Thirteen (52%) patients of 25 had received adjuvant treatment: ten patients had taken only radiotherapy, one had whole pelvic radiation (WPI) with para-aortic radiation, and two had both radiotherapy and chemotherapy. Postoperative treatment was not absolutely consistent; however, it mainly consisted of adjuvant WPI. WPI consisted of megavoltage photonic irradiation by 6 MeV linear accelerator delivering about 4 500 to 5 000 cGy to whole pelvis over 4 to 5 weeks in daily fractions of 180 to 200 cGy through AP-PA opposed fields or four-field “box” technique. This was followed by brachytherapy using two iridium-192 insertions each delivering 6.5 Gy high dose rate brachytherapy or 20 Gy low dose rate brachytherapy to the vaginal surface.
Table 1
Patients’ clinical characteristics
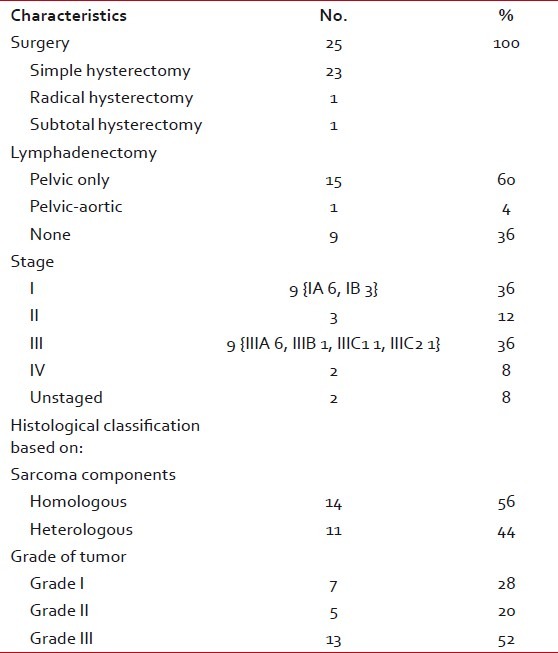
Table 2
Presenting symptoms

Ten patients had WPI, one had WPI with para-aortic radiation because of positive para-aortic nodes, and two patients had radiation therapy along with chemotherapy. Of the two patients who received both radiotherapy and chemotherapy, one patient had coexistent stage IIIB ovarian serous papillary adenocarcinoma and other patient had stage IVB disease with only one-sided inguinal node metastases. Patient with inguinal node metastases underwent groin dissection along with surgical staging and postoperative three cycles of cisplatin and epirubicin followed by WPI. This was the only case of advanced disease with long disease-free period of 52 months after which she was lost to follow-up.
Table 3 presents summary of postoperative treatment and recurrence. Of the 13 patients who had taken adjuvant treatment, recurrence was recorded in total five (38.5%) patients, of which four patients had only postoperative radiation as adjuvant therapy and one patient had chemotherapy followed by radiation therapy because of coexistent stage IA uterine carcinosarcoma and stage IIIB serous papillary adenocarcinoma of ovary. Forty percent (4/10) of patients who had taken only postoperative radiotherapy developed recurrence. Three of these four patients had high-grade tumors and all four of them had distant metastases. Among the group of 12 patients who had not taken any adjuvant treatment, five patients were lost after 1 to 6 months, two patients had significant residual disease, and five (100%) patients had recurrence. So, almost all patients who had not taken adjuvant treatment developed recurrence and in none of them lymphadenectomy was done on account of advanced stage.
Table 3
Summary of postoperative treatment and recurrence
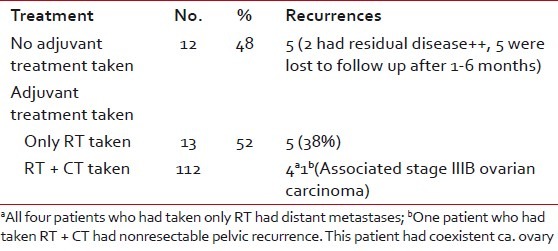
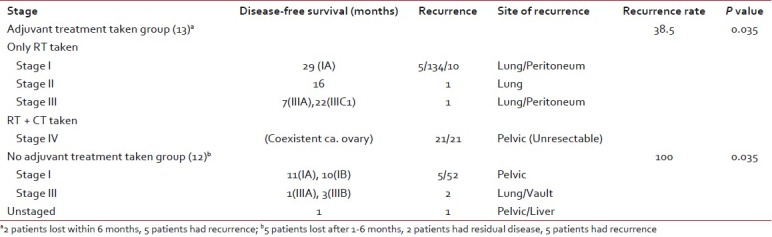
Table 4 shows stage-wise analysis of recurrence in the two groups, who had taken and not taken adjuvant treatment. Three patients with advanced stages (stageIII-2, unstaged-1) who had not taken adjuvant treatment developed recurrence within 1 to 3 months and two with stage I disease developed recurrence after period of 10 to 11 months. Among the adjuvant treatment taken group, one patient of stage IA had recurrence after 29 months, another of stage II after 16 months, and two patients with stage III after 7 and 22 months, respectively. One patient of stage IA had a very early recurrence, i.e., after 5 months, which can be attributed to coexistent ovarian carcinoma.
Table 4
Stage-wise analysis of recurrence
Sixty percent (15) of patients had undergone total abdominal hysterectomy with bilateral salpingo-oophorectomy with bilateral pelvic lymph node dissection. Of the ten patients who did not underwent lymphadenectomy, two patients had significant residual disease and both of them expired within six months of surgery, three patients had taken adjuvant treatment and all of them had DFS of at least one year, rest of all five patients were of advanced stage and developed recurrence. Of these three patients who did not develop recurrence, two were of stage IA and third was in stage IIIA.
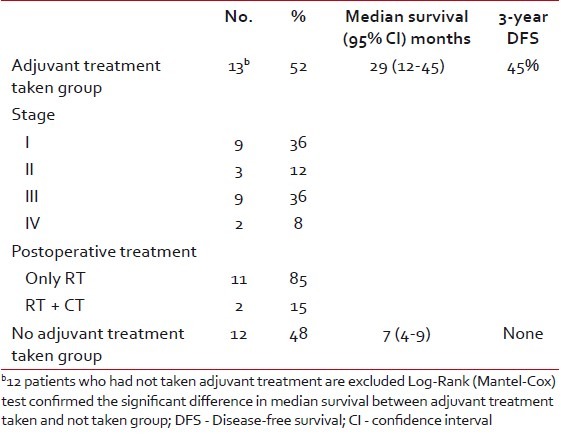
Table 5 presents the median and 3-year survival time according to postoperative treatment. The overall 3-year DFS of 13 patients who had taken adjuvant therapy was 40%. The differences in survival of patients with stage I and stage II-IV was statistically significant (P value=0.035).
Table 5
Median and 3-year disease-free survival according to stage and postoperative treatment
The difference in survival of 13 patients who had taken adjuvant treatment was significantly more than the group who had not taken adjuvant therapy (P=0.025). However, these adjuvant treatment modalities had borderline statistical significance on overall survival of patients (P=0.075) [Figure 1].
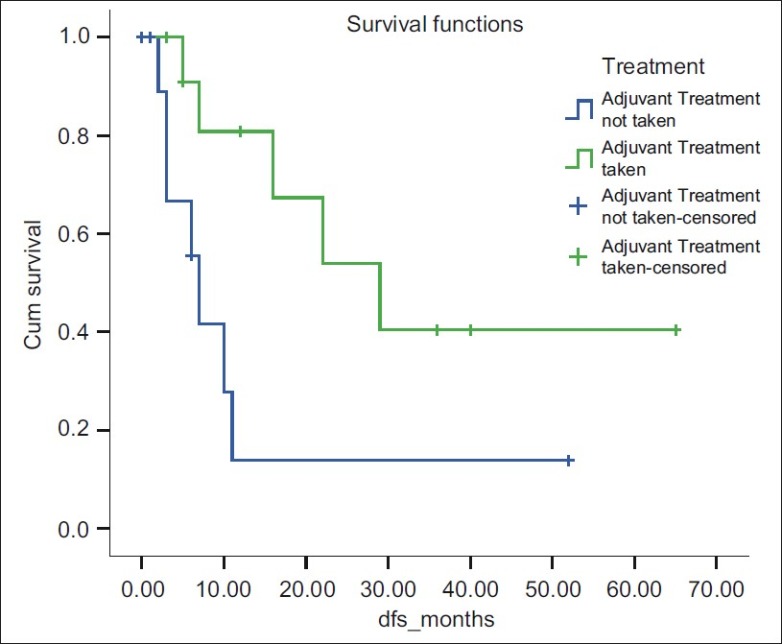
| Figure 1: Disease-free survival for 3 year was 40% for adjuvant treatment taken group
DISCUSSION
Our data indicated that the difference in survival of 13 patients who had taken adjuvant treatment was significantly more than the group who had not taken adjuvant therapy (P=0.025). The radiotherapy as treatment modality was associated with DFS period of three years in 40% of patients. Of the ten patients who had taken only radiotherapy as adjuvant treatment, four had recurrence after a median DFS period of 18.5 months. All these recurrences were distant metastases which prove the efficacy of radiotherapy to prevent locoregional recurrence, though not statistically. Many studies indicate that patients treated by adjuvant WPI alone have a significant reduction of recurrences within the radiation treatment field.[9] Considering distant failures, which constituted the primary site of recurrence accounting for the compromised 5-year survival rates for both early and advanced disease, systemic therapy after initial surgical extirpation of uterus and adnexal structures would appear prudent.
There are no prospective studies indicating that adjuvant radiotherapy or chemoradiation confer a survival benefit in patients with uterine carcinosarcoma. However, because the risk of recurrence is high even in early stage disease, these treatment modalities are often used. Adjuvant radiotherapy has been frequently used in management of uterine sarcomas. Results with regard to improvement of survival are inconsistent. Some authors have found an improvement,[10,11] while others reported no such effect on survival.[12,13] Our data showed that adjuvant treatment modalities had borderline statistical significance on overall survival of patients (P=0.075).
The limitations of our study are the small sample size in both postoperative treatment category and all inherent deficiencies of a retrospective study including the lacking of some data. Our series is too small for meaningful statistical analysis of correlation between adverse pathological findings, other than stage, treatment type, and survival.
Our results seem to indicate that in this highly aggressive malignancy, further exploration of potential outcome benefits of postoperative treatment, especially chemoradiation, is warranted in larger group of patients after comprehensive surgical staging.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Figure 1: Disease-free survival for 3 year was 40% for adjuvant treatment taken group


 PDF
PDF  Views
Views  Share
Share

