Tyrosine Kinase Inhibitor versus Physician Choice Chemotherapy in Second-Line Epidermal Growth Factor Receptor Mutation Non-Small Cell Lung Cancer: Post hoc Analysis of Randomized Control Trial
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(04): 493-498
DOI: DOI: 10.4103/ijmpo.ijmpo_219_17
Abstract
Background: There is a paucity of prospective data for patients who progressed after first-line tyrosine kinase inhibitor (TKI) or pemetrexed doublet among epidermal growth factor receptor (EGFR) mutation-positive metastatic non-small cell lung cancer (NSCLC). Aim: The aim of the study was to evaluate the outcome of second-line therapy in patients who progressed on TKI or pemetrexed doublet in EGFR mutation-positive NSCLC. Objective: The objective of the study was to calculate response rates, progression-free survival (PFS), and overall survival (OS) of patients receiving second-line therapy in EGFR mutation NSCLC. Materials and Methods: Post hoc analysis of second-line therapy among patients enrolled in randomized control trial comparing TKI versus pemetrexed doublet in EGFR mutation NSCLC. Kaplan–Meir statistics were used for PFS and OS. Impact of variables was measured with Log-rank test. Results: One hundred and eighty-seven patients who progressed on first-line therapy and received second-line agents were analyzed. Male:female: 110 (56.3%):77 (41.2%). One hundred and thirteen patients received gefitinib, while 74 received chemotherapy. Response rate (complete response + partial response) was 53%-versus 24%-in gefitinib versus chemotherapy group (RECIST v1.1). PFS was 7.4 months versus 4.4 months (P = 0.001), while OS was 14 months versus 9.7 months (P = 0.007), in gefitinib versus chemotherapy group, respectively. Response to TKI significantly improves PFS (10.8 months vs. 3.9 months, P = 0.001) and OS (21.4 months vs. 8.9 months, P = 0.03). Rash, pruritus, dry skin, fatigue, diarrhea, and paronychia were common toxicities of TKI. Conclusion: Second-line TKI improves outcome in EGFR mutation-positive NSCLC who progressed after first-line chemotherapy. Response to therapy, whether with TKI or chemotherapy, favorably impacts outcomes.
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: There is a paucity of prospective data for patients who progressed after first-line tyrosine kinase inhibitor (TKI) or pemetrexed doublet among epidermal growth factor receptor (EGFR) mutation-positive metastatic non-small cell lung cancer (NSCLC). Aim: The aim of the study was to evaluate the outcome of second-line therapy in patients who progressed on TKI or pemetrexed doublet in EGFR mutation-positive NSCLC. Objective: The objective of the study was to calculate response rates, progression-free survival (PFS), and overall survival (OS) of patients receiving second-line therapy in EGFR mutation NSCLC. Materials and Methods: Post hoc analysis of second-line therapy among patients enrolled in randomized control trial comparing TKI versus pemetrexed doublet in EGFR mutation NSCLC. Kaplan–Meir statistics were used for PFS and OS. Impact of variables was measured with Log-rank test. Results: One hundred and eighty-seven patients who progressed on first-line therapy and received second-line agents were analyzed. Male:female: 110 (56.3%):77 (41.2%). One hundred and thirteen patients received gefitinib, while 74 received chemotherapy. Response rate (complete response + partial response) was 53%-versus 24%-in gefitinib versus chemotherapy group (RECIST v1.1). PFS was 7.4 months versus 4.4 months (P = 0.001), while OS was 14 months versus 9.7 months (P = 0.007), in gefitinib versus chemotherapy group, respectively. Response to TKI significantly improves PFS (10.8 months vs. 3.9 months, P = 0.001) and OS (21.4 months vs. 8.9 months, P = 0.03). Rash, pruritus, dry skin, fatigue, diarrhea, and paronychia were common toxicities of TKI. Conclusion: Second-line TKI improves outcome in EGFR mutation-positive NSCLC who progressed after first-line chemotherapy. Response to therapy, whether with TKI or chemotherapy, favorably impacts outcomes.
Introduction
Platinum doublet chemotherapy has been the historical backbone of therapy for advanced metastatic non-small cell lung cancer (NSCLC).[1] Pemetrexed platinum doublet is accepted standard of care for chemotherapy-naive nonsquamous NSCLC.[2] However, recently, use of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive metastatic NSCLC has shown to significantly improve progression-free survival (PFS) when used upfront compared to chemotherapy.[3] [4] [5] [6] [7] [8] [9] [10] [11] However, caveat to these studies is that, except for LUX-Lung 3, all have nonpemetrexed doublet in comparator arm and have few patients from Indian Subcontinent.[12] [13] Moreover, none had maintenance pemetrexed in control arm, which is now an accepted standard worldwide. We had earlier reported outcomes of Phase III randomized trial comparing first-line TKI and pemetrexed doublet in EGFR mutation-positive NSCLC.[14] We further report post hoc analysis of outcomes and toxicities of patients who progressed after first-line therapy including maintenance pemetrexed and managed to receive second-line chemotherapy or TKIs in EGFR mutation positive NSCLC.
Materials and Methods
Our group from tertiary cancer center at Mumbai, India, has initially reported outcomes of Phase III, double-arm, single-center, open-label randomized control trial comparing gefitinib versus pemetrexed platinum doublet in first-line EGFR mutation-positive nonsquamous advanced metastatic NSCLC. Out of 290 patients enrolled, 257 progressed after first-line therapy. One hundred and eighty-seven out of these 257 went on to receive second-line physician choice agents; remaining others were not eligible due to various reasons such as poor performance status, progressive brain metastasis, asymptomatic progression, patient not willing for further therapy, and clinician decision [CONSORT diagram [Figure 1]. This study was approved by the Institutional Ethics Committee of our Institute.
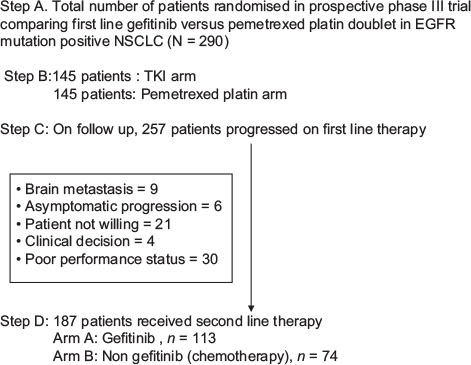
| Figure 1:CONSORT diagram: Schematic representation of patients on prospective randomised trial who received second line therapy after progression to first line
Demographic and clinical profile of patients who went on to receive physician choice second-line therapy was recorded including age, sex, performance status before initiation of second-line therapy, smoking status, EGFR mutation, and tobacco chewing. For EGFR mutation analysis, DNA was extracted from formalin fixed, paraffin-embedded tumor block and amplified for exon 18, 19, and 21 using nested PCR which has been standardized and validated at our institute molecular laboratory.[15] [16]
As per physician choice, any of the following schedules of drug administration was followed:
-
Gefitinib 250 mg daily per oral until progressive disease, intolerable toxicity, or other approved criteria for discontinuation
-
Pemetrexed 500 mg/m2 intravenous over 10 min with or without carboplatin (area under the curve [AUC] =5) over 60 min intravenous infusion, with appropriate antiemetic prophylaxis along with B12 (1000 mcg intramuscularly 1 week before chemotherapy and repeated every 12 weeks) and folic acid (5 mg once a day daily) continued till 3 weeks after last dose of pemetrexed
-
Weekly paclitaxel at 100 mg/m2 infused over 60 min, continued till progressive disease, Grade II neuropathy or other intolerable toxicity
-
Gemcitabine 1000 mg/m2 infused over 20 min D1 and D8, with or without cisplatin 75 mg/m2 over 60 min with adequate intravenous hydration or carboplatin (AUC = 5) over 60 min intravenous infusion
Response evaluation was done at every 12 weeks with contrast-enhanced computerized tomography of chest and associated metastatic sites in patient receiving second-line therapy including gefitinib and chemotherapy, recorded and compared as per RECIST criteria version 1.1. Toxicity with the use of second-line agents was prospectively recorded at every cycle visit of chemotherapy and every 2 weeks for the first 2 months for TKIs and thereafter monthly. Patients were followed through clinical charts in each outpatient department visit during and after completion of therapy including telephonic calls.
PFS was defined as time interval from the start of second-line agent to objective progressive disease and change in treatment or death from any cause. Overall survival (OS) was defined as time interval from start of second-line agent till death from any cause. PFS and OS were calculated as per Kaplan–Meir statistics. Log-rank test was used to compare the impact of several prognostic factors such as age, sex, smoking, tobacco use, EGFR mutation, performance status, response rates, on PFS and OS.
Results
Patients were enrolled in prospective randomized Phase III trial from February 2012 to April 2016, all having EGFR classical mutation and metastatic advanced nonsquamous NSCLC. One hundred and eighty-seven out of 257 (73%) patients, who progressed after first-line therapy, went on to receive physician choice second-line therapy. Gefitinib was the most common agent used (n = 113) followed by pemetrexed platinum doublet (n = 58), weekly paclitaxel (n = 6), pemetrexed alone (n = 1), and other agents (n = 9). Male patients and smokers were 110 and 39, respectively. One hundred and forty-three (76%) patients had a good performance status (PS 0–1), whereas 14 (7.5%) had PS 3 before starting second-line agents. Eighty-eight out of one hundred and eighty-seven (47%) patients underwent re-biopsy after progression on first-line agents. Most common site of biopsy was lung (n = 79), followed by extrathoracic site (n = 6) and lymph node (n = 3).
Of all patients re-biopsied, three patients had nonadenocarcinoma histology, with squamous cell, small cell, and poorly differentiated carcinoma each, while remaining 77 had similar adenocarcinoma histology, and in 8 patients, sample was not representative [Table 1]. Gefitinib was used in 36/64, 76/116, and 1/4 patients with exon 21, exon 19, and exon 18 mutation, respectively, while remaining patients received nongefitinib chemotherapeutic agents.
|
Profile (n=187) |
Characteristics |
Remarks (%) |
|---|---|---|
|
SD – Stable disease; PD – Progressive disease; PR – Partial response; CR – Complete response; PS – Performance status |
||
|
Age |
Median 54 years |
Range 27-76 year |
|
Sex |
||
|
Male |
110 |
59 |
|
Female |
77 |
41 |
|
Smoking |
||
|
Yes |
39 |
21 |
|
No |
148 |
79 |
|
Tobacco chewing |
||
|
Yes |
75 |
40 |
|
No |
112 |
60 |
|
EGFR mutation |
||
|
Exon 21 |
64 |
34 |
|
Exon 19 |
119 |
64 |
|
Exon 18 |
4 |
2 |
|
PS |
||
|
PS 0-1 |
143 |
76 |
|
PS 2 |
30 |
16 |
|
PS 3 |
14 |
7.5 |
|
Second line agents |
||
|
Gefitinib |
113 |
60 |
|
Others (nongefitinib) |
74 |
40 |
|
Pemetrexed platinum |
58 |
31 |
|
Other chemotherapy |
16 |
9 |
|
Response rates |
Gefitinib |
Nongefitinib |
|
SD+ PD |
53 |
56 |
|
CR + PR |
60 |
18 |
|
Factors |
Group |
PFS (months) |
OS (months) |
||||
|---|---|---|---|---|---|---|---|
|
Gefitinib |
Nongefitinib |
P |
Gefitinb |
Nongefitinib |
P |
||
|
#OS is significantly better in nonsmokers compared to smokers in gefitinib group, P=0.03, $CR+PR responses had significantly better PFS and OS in both gefitinb and nongefitinib group. PS – Performance status, EGFR mut – Epidermal Growth Factor receptor mutation type; SD – Stable disease; PD – Progressive disease; PR – Partial response; CR – Complete response; NS – Not significant, OS – Overall survival |
|||||||
|
Age (years) |
≤54 |
7.4 |
4.7 |
NS/NS |
15.2 |
10.7 |
NS/NS |
|
>54 |
8.3 |
4.9 |
13.4 |
7.4 |
|||
|
Sex |
Male |
6.5 |
4.1 |
NS/NS |
13.7 |
8.86 |
NS/NS |
|
Female |
8.7 |
4.5 |
15.2 |
11.5 |
|||
|
Smoking |
Yes |
6.2 |
3.6 |
NS/NS |
11.1# |
9.7 |
0.03/NS |
|
No |
8.0 |
4.4 |
15.2# |
9.7 |
|||
|
Tobacco |
Yes |
6.5 |
4.5 |
NS/NS |
13.8 |
11.2 |
NS/NS |
|
No |
7.6 |
4.4 |
15.2 |
9.5 |
|||
|
EGFR mut |
Exon 21 |
5.7 |
4.1 |
NS/NS/NS |
11.5 |
8.2 |
NS/NS/NS |
|
Exon 19 |
8.0 |
4.5 |
14.8 |
9.7 |
|||
|
Exon 18 |
8.4 |
2.3 |
14.0 |
9.1 |
|||
|
PS |
0-1 |
7.5 |
5.2 |
NS/NS |
15.7 |
11.3 |
NS/NS |
|
2-3 |
6.1 |
4.3 |
11.5 |
7.2 |
|||
|
Response |
SD+PD |
3.9 |
3.8 |
0.00/0.07$ |
8.9 |
8.8 |
0.00/0.03$ |
|
CR+PR |
10.8 |
6.2 |
21.4 |
11.7 |
|||
|
Toxicity |
Gefitinib group (n=113) |
Nongefitinib group (n=74) |
||||||
|---|---|---|---|---|---|---|---|---|
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 1 |
Group 2 |
Group 3 |
Group 4 |
|
|
SGPT – Serum glutamate pyruvate transaminase; SGOT – Serum glutamate-oxaloacetate transaminase |
||||||||
|
Anaemia |
43 |
12 |
5 |
0 |
28 |
15 |
3 |
0 |
|
Thrombocytopena |
7 |
4 |
1 |
1 |
8 |
3 |
2 |
1 |
|
Neutropenia |
1 |
4 |
0 |
0 |
10 |
2 |
2 |
4 |
|
SGOT increased |
31 |
4 |
2 |
0 |
14 |
2 |
0 |
0 |
|
SGPT increased |
31 |
5 |
5 |
0 |
18 |
4 |
0 |
0 |
|
Hypoalbuminemia |
14 |
12 |
2 |
0 |
15 |
6 |
0 |
0 |
|
Diarrhea |
14 |
7 |
3 |
0 |
7 |
6 |
6 |
0 |
|
Skin rash |
34 |
22 |
8 |
0 |
20 |
4 |
2 |
0 |
|
Paronychia |
3 |
2 |
1 |
0 |
1 |
1 |
0 |
0 |
|
Mucositis |
8 |
1 |
0 |
0 |
11 |
0 |
2 |
0 |
|
Anorexia |
39 |
4 |
1 |
0 |
21 |
4 |
0 |
0 |
|
Pruritus |
33 |
2 |
0 |
0 |
16 |
4 |
0 |
0 |
|
Nausea |
8 |
2 |
0 |
0 |
9 |
2 |
0 |
0 |
|
Vomiting |
8 |
3 |
1 |
0 |
4 |
0 |
2 |
0 |
|
Fatigue |
45 |
10 |
2 |
0 |
24 |
15 |
3 |
0 |
|
Constipation |
9 |
0 |
1 |
0 |
11 |
2 |
0 |
0 |
|
Bilirubin increase |
5 |
1 |
0 |
0 |
6 |
0 |
0 |
0 |
|
Creatinine increase |
6 |
3 |
0 |
0 |
6 |
3 |
0 |
0 |
|
Hypokalemia |
6 |
1 |
2 |
0 |
6 |
2 |
0 |
0 |
|
Pneumonitis |
1 |
2 |
0 |
0 |
1 |
1 |
0 |
1 |
|
Dry skin |
38 |
11 |
1 |
0 |
13 |
3 |
1 |
0 |

| Figure 1:CONSORT diagram: Schematic representation of patients on prospective randomised trial who received second line therapy after progression to first line
References
- D’Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, Shepherd FA. et al. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: A meta-analysis of the published literature. J Clin Oncol 2005; 23: 2926-36
- Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C. et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26: 3543-51
- Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM. et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: A meta-analysis. J Natl Cancer Inst 2013; 105: 595-605
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C. et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015; 26: 1877-83
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239-46
- Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z. et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883-9
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947-57
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V. et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29: 2866-74
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J. et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121-8
- Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H. et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013; 24: 54-9
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380-8
- Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T. et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327-34
- Yang JC, Hirsh V, Schuler M, Yamamoto N, O’Byrne KJ, Mok TS. et al. Symptom control and quality of life in LUX-lung 3: A phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3342-50
- Patil VM, Noronha V, Joshi A, Choughule AB, Bhattacharjee A, Kumar R. et al. Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma. ESMO Open 2017; 2: e000168
- Chougule A, Prabhash K, Noronha V, Joshi A, Thavamani A, Chandrani P. et al. Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One 2013; 8: e76164
- Noronha V, Prabhash K, Thavamani A, Chougule A, Purandare N, Joshi A. et al. EGFR mutations in Indian lung cancer patients: Clinical correlation and outcome to EGFR targeted therapy. PLoS One 2013; 8: e61561
- Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J. et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589-97
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY. et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 trial) [corrected]. J Clin Oncol 2003; 21: 2237-46
- Kris MG, Natale RB, Herbst RS, Lynch Jr. TJ, Prager D, Belani CP. et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA 2003; 290: 2149-58
- Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. SIGN Study Group. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anticancer Drugs 2006; 17: 401-9
- Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL. et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet 2008; 372: 1809-18
- Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K. et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008; 26: 4244-52
- Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH. et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res 2010; 16: 1307-14
- Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R. et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: Data from the randomized phase III INTEREST trial. J Clin Oncol 2010; 28: 744-52


 PDF
PDF  Views
Views  Share
Share

