Trastuzumab Emtansine: Antibody-drug Conjugate in Treatment of Human Epidermal Growth Factor Receptor-2-Positive Metastatic Breast Cancer
CC BY-NC-ND 4.0 Indian J Med Paediatr Oncol 2018; 39(01): 79-87
DOI: DOI: 10.4103/ijmpo.ijmpo_53_17
Abstract
The human epidermal growth factor receptor-2 (HER2)-targeted therapies have improved clinical outcomes for patients at any stage of HER2-positive breast cancer (BC). Trastuzumab, a monoclonal antibody that targets the HER2 receptor on BC cells, showed improved survival in metastatic BC (MBC). However, resistance to therapy arises in the majority of patients with advanced disease. Antibody?drug conjugate (ADC) is a relatively new development to deliver cytotoxic drugs specifically to cancer cells. Trastuzumab emtansine (T-DM1) is a HER2-targeted ADC, composed of trastuzumab, a stable thioether linker, and the potent cytotoxic agent, emtansine (DM1, derivative of maytansine). T-DM1 has been approved for use in patients with MBC who have failed prior therapy with trastuzumab and a taxane. Dose finding Phase I study established the maximum tolerated dose at 3.6 mg/kg every 3 weeks. Phase I and II studies of T-DM1 have shown clinical activity and a favorable safety profile in HER2-positive MBC patients. The Phase III randomized EMILIA and TR3RESA trials demonstrated that T-DM1 significantly improves progression-free and overall survival in pretreated HER2-positive MBC patients. Nausea and fatigue are most commonly reported adverse drug reactions with T-DM1 and cardiac toxicity comparable with standard of care therapies. The drug is well tolerated in most patients, with a predictable pharmacokinetic profile and minimal systemic exposure to free cytotoxic DM1. T-DM1 has emerged as an effective therapeutic option for the management of patients with HER2-positive MBC.
Keywords
Drug conjugate - human epidermal growth factor receptor-2 - metastatic breast cancer - Trastuzumab emtansine
Publication History
23 June 2021
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The human epidermal growth factor receptor-2 (HER2)-targeted therapies have improved clinical outcomes for patients at any stage of HER2-positive breast cancer (BC). Trastuzumab, a monoclonal antibody that targets the HER2 receptor on BC cells, showed improved survival in metastatic BC (MBC). However, resistance to therapy arises in the majority of patients with advanced disease. Antibody drug conjugate (ADC) is a relatively new development to deliver cytotoxic drugs specifically to cancer cells. Trastuzumab emtansine (T-DM1) is a HER2-targeted ADC, composed of trastuzumab, a stable thioether linker, and the potent cytotoxic agent, emtansine (DM1, derivative of maytansine). T-DM1 has been approved for use in patients with MBC who have failed prior therapy with trastuzumab and a taxane. Dose finding Phase I study established the maximum tolerated dose at 3.6 mg/kg every 3 weeks. Phase I and II studies of T-DM1 have shown clinical activity and a favorable safety profile in HER2-positive MBC patients. The Phase III randomized EMILIA and TR3RESA trials demonstrated that T-DM1 significantly improves progression-free and overall survival in pretreated HER2-positive MBC patients. Nausea and fatigue are most commonly reported adverse drug reactions with T-DM1 and cardiac toxicity comparable with standard of care therapies. The drug is well tolerated in most patients, with a predictable pharmacokinetic profile and minimal systemic exposure to free cytotoxic DM1. T-DM1 has emerged as an effective therapeutic option for the management of patients with HER2-positive MBC.
Keywords
Drug conjugate - human epidermal growth factor receptor-2 - metastatic breast cancer - Trastuzumab emtansine
Introduction
Breast cancer (BC) is the second most common cancer worldwide and second leading cause of cancer-related death in women.[1] Research over the past 3 decades has led to a better insight into multifaceted molecular heterogeneity of the disease. The discovery of human epidermal growth factor receptor 2 (HER2) (also known as epidermal growth factor receptor or Erb-B), a membrane tyrosine kinase and oncogene, was one such important finding.[2],[3] Slamon et al. showed that amplification of HER2 gene occurs relatively infrequently in BC, and that it is associated with disease relapse and reduced overall patient survival.[2] The HER2 proteins are involved in promoting cell growth through activation of the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt)-mammalian target of rapamycin and Ras-Raf-MEK-Erk1/2 pathways, resulting in tumor growth and progression.[4],[5]
BC cells can have up to 25 50 copies of the HER2 gene (HER2 amplification) and up to 40 100-fold increase in HER2 protein resulting in two million receptors expressed at the tumor cell surface (HER2 overexpression).[6] HER-2 is amplified in 15 % of human primary BC and is significant predictor of both overall survival (OS) and time to relapse in patients with BC.[7] The identification of HER2 in BC pathogenesis has led to the development of therapies targeting this receptor.[8]
Trastuzumab (Herceptin ; Genentech, South San Francisco, CA, USA), the first monoclonal antibody developed to target HER2, received US Federal Drug Authority approval in 1998 for the treatment of HER2-positive metastatic BC (MBC) in combination with paclitaxel for first-line treatment.[9] Trastuzumab was shown to significantly improve the time to progression and OS of patients with metastatic HER2-positive BC.[10],[11]
Despite the significant efficacy of trastuzumab-based therapy, 50% of patients progress within 1 year.[10],[11] Lapatinib, an orally administered small molecule inhibitor of the HER1 and HER2 tyrosine kinases, was found to be superior in combination with capecitabine compared with capecitabine alone, in the treatment of HER2-positive MBC that had progressed after trastuzumab-based therapy.[12] Although this combination therapy provided patients with trastuzumab-resistant disease, an additional treatment option, only 29% of patients showed clinical benefit (complete response, partial response, or stable disease lasting at least 6 months), and half of patients had disease progression at 6.2 months.[13]
Diarrhea is a well-known side effect and a dose-limiting factor associated with lapatinib plus capecitabine treatment. Despite availability of treatment guidelines for the management of lapatinib capecitabine-associated diarrhea, it still represents a significant limitation in the optimal regimen administration in many patients. This frequently has a negative impact on patients' quality of life and efficacy of drug in daily clinical practice.[14]
In 2013, the FDA approved the first successful HER2-targeted antibody drug conjugate (ADC), trastuzumab emtansine (T-DM1; Kadcyla ; Genentech), for the treatment of HER2-positive trastuzumab-pretreated MBC.[15] In this review, we will discuss the pharmacology, efficacy, and tolerability of T-DM1 in HER2-positive MBC. A search of published medical literature was performed following the principles of evidence-based medicine. The search strategy included a search using the keywords: T-DM1, HER2+ve BC, HER2 targeted therapy, MBC in PubMed, Medscape, ClinicalTrials.gov, in addition to older studies identified by the literature reviews were reviewed.
Trastuzumab Emtansine-Human Epidermal Growth Factor Receptor-2-Targeted Antibody–drug Conjugate
ADCs are relatively new drugs and are designed to deliver cytotoxic drugs specifically into cancer cells,[16] thereby creating a more favorable therapeutic window for cytotoxic agents than that would be achieved by a free cytotoxic agent.[17] The key components of an ADC are the cytotoxic agent, a monoclonal antibody targeting a tumor-enriched or tumor-specific antigen, and a linker; covalently binding these components together.[18]
T-DM1, first ADC targeting the HER2 receptor, is a conjugate of trastuzumab through a non-reducible thioether linker (N-succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate [SMCC]) and a cytotoxic moiety (emtansine, derivative of maytansine [DM1]).[19]
Trastuzumab
Trastuzumab component of T-DM1 binds to subdomain 4 of HER2 receptor and exerts its own antitumor effects. The HER2-DM1 complex is then endocytosed and ultimately fused with a lysosome where it undergoes proteolytic degradation with release of the active DM1.[20]
Derivative of maytansine
DM1 is the derivative of maytansine (C34H46 ClN3O10), a benzoansamacrolide collected from plants and mosses. Maytansine is a potent microtubule-targeted compound, considered to have a high affinity for tubulin located at the ends of microtubules. The suppression of microtubule dynamics causes cells to arrest in the G2/M phase of the cell cycle, ultimately resulting in cell death by apoptosis.[21] In vitro studies demonstrate that on a molar basis across a range of cancer cell lines, DM1 is 24- to 270-fold more potent than paclitaxel, 180- to 4000-fold more potent than doxorubicin, and 100 fold more potent than vincristine (Vinca alkaloids).[22],[23],[24]
Maytansine had been extensively evaluated in Phase I and II clinical trials in humans, but side effects mainly gastrointestinal and neurologic toxicities and lack of tumor specificity have prevented its successful clinical development.[25],[26],[27] However, DM1, a derivative of maytansine, was selected for use in T-DM1, owing to high potency, excellent stability, in addition to the acceptable solubility of maytansine in aqueous solutions.[22],[26]
Thioether linker
The linker should stabilize the ADC in circulation, and once the compound enters the cell, it should liberate the cytotoxic agent either in a pH-dependent manner or by disulfide reaction.[28] T-DM1 is the first clinically developed ADC that uses the noncleavable linker. The advantage of noncleavable linker is that it undergoes proteolytic degradation once internalized and has better stability while in circulation.[19]
The conjugation of linker to trastuzumab is multistep process, first step is reaction of SMCC with the amino side chain of a lysine residue to form an amide bond at pH 7 9. Subsequently, the maleimide moiety undergoes a Michael-type addition with thiols at pH 6.5 7.5 to form thioether bonds with the cytotoxic agent resulting in an average 3.5 molecules per trastuzumab antibody (different drug antibody ratio).[21]
Trastuzumab Emtansine - mechanisms of Action
The mechanism of action (MOA) of T-DM1 is twofold [Figure 1].
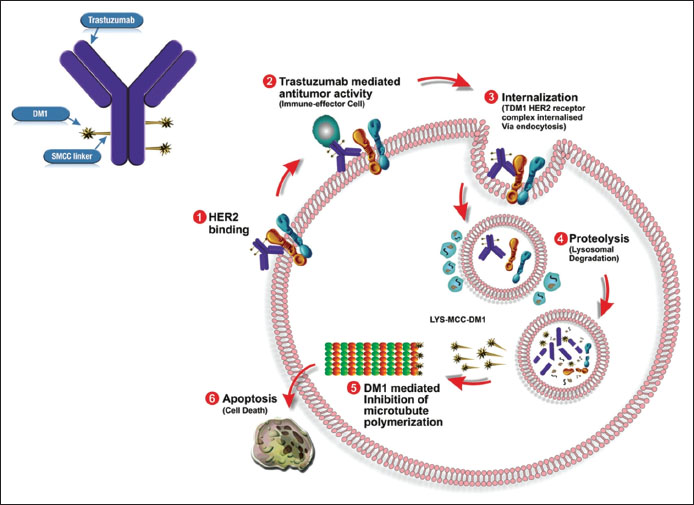
| Figure.1Trastuzumab emtansine - mechanism of action
First, T-DM1 has been shown to retain the MOA of unconjugated trastuzumab including inhibition PI3K/AKT pathway, inhibition of HER-2 shedding, and Fc receptor mediated engagement of immune cells, which may result in antibody-dependent cellular cytotoxicity.[23] Moreover, trastuzumab-mediated effect should not be underestimated and is particularly of importance, when target cells do not undergo rapid apoptotic death caused by DM1.[18]
Second, binding of T-DM1 to HER2 triggers entry of the HER2-T-DM1 complex into the cell through receptor-mediated endocytosis and ultimately fused with a lysosome where it undergoes proteolytic degradation.[29],[30],[31] As nonreducible linker is stable in the circulation and the tumor microenvironment, conjugates are efficiently degraded in lysosomes to yield metabolites consisting of the intact maytansinoid drug and linker attached to lysine.[19],[31] Subsequent to release from lysosome, microtubule assembly is inhibited by DM1-containing metabolites, finally causing cell death.[32] The primary active metabolite, lysine SMCC DM1, is a charged molecule, and relatively membrane impermeable, reducing the possibility that the DM1 entering a neighboring cell.[22]
Trastuzumab Emtansine Pharmacokinetics
T-DM1 appears quite stable in circulation, as very low levels of free DM1 were reported to be present in plasma samples from patients treated with T-DM1.[32]
Lu et al. evaluated s erum samples collected from 671 patients with HER2-positive locally advanced or MBC who received single-agent T-DM1 in five Phase I to Phase III studies. The results from the study showed terminal half-life of 3.94 days, with clearance of 0.676 L/day and a central volume of 3.127 L. Age, race, region, and renal function had no influence on pharmacokinetic of T-DM1.[33] In Phase I study of HER2-positive MBC patients with normal or reduced hepatic function, Li et al. reported that no increase in the systemic concentration of DM1 was observed in patients with mild or moderate hepatic impairment, compared to patients with normal hepatic function.[34] T-DM1 is neither an inducer nor inhibitor of CYP isoform. There was no accumulation or tissue retention by day 14 and 80% of the drug was excreted in feces and a small fraction in urine.[35]
Clinical Efficacy
Dose finding studies
T-DM1 was initially evaluated as a single agent in a Phase I dose escalation study in patients with trastuzumab-refractory HER2-positive advanced BC. Both weekly and 3-weekly schedules were tested. The 3-weekly dosing cohort was enrolled first. A total of 24 patients received intravenous T-DM1 doses at 0.3 mg/kg to 4.8 mg/kg every 3 weeks.[18] Grade IV thrombocytopenia was dose limiting at 4.8 mg/kg. The investigators deemed the maximum tolerated dose (MTD) to be 3.6 mg/kg. Response rate in these heavily pretreated patients with measurable disease at MTD was 44%.[18]
Phase II studies
Burris et al. conducted a Phase II clinical trial (TDM 4258 g) [Table 1] in 112 patients with HER2-positive MBC with tumor progression after prior HER2-directed therapy. By independent review, the objective response rate (ORR) was 26%.[32]{Table 1}
|
Trial and reference |
Year |
Study population |
Patients (n) |
Regimen/ treatment groups |
End points |
ORR |
CR |
CBR |
Median (months) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Primary |
Secondary |
DOR |
PFS |
|||||||||
|
aDefined as CR plus partial response plus stable disease 6 months. CBR Clinical benefit rate; CR Complete response; DOR Duration of response; HER2 Human epidermal growth factor receptor 2; IRF Independent radiologic facility; MBC Metastatic breast cancer; NR Not reported; ORR Objective response rate; PFS Progression-free survival; q3w Every-3-week; T Trastuzumab; T-DM1 Trastuzumab emtansine; IV Intravenous |
||||||||||||
|
Burris et al. (TDM4258g)[32] |
2011 |
Previously treated with chemotherapy and progressed on HER2-targeted therapy |
112 |
T-DM1; 3.6 mg/kg IV, q3w |
ORR by IRF, safety, and tolerability |
ORR by investigator review, DOR, PFS by IRF |
26% |
3.6% |
NR |
9.4 |
4.6 |
|
|
Krop et al. (TDM4374g)[36] |
2012 |
Previously treated with anthracycline, a taxane, and capecitabine, plus lapatinib, and T for MBC |
110 |
T-DM1; 3.6 mg/kg IV, q3w |
ORR by IRF, safety, and tolerability |
CBR, DOR, PFS |
34.5% |
0% |
48.2%a |
7.2 |
6.9 |
|
|
Trial and reference |
Year |
Study population |
Patients (n) |
Regimen/ treatment groups |
End points |
ORR (%) |
Median (months) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Primary |
Secondary |
OS |
DOR |
PFS |
|||||||
|
a83% of the patients received HER2-targeted therapy and 17% received single-agent chemotherapy, as part of their regimen; bPertuzumab placebo. BID Twice daily; CBR Clinical benefit rate; D Docetaxel; DOR Duration of response; HER2 Human epidermal growth factor receptor 2; IRF Independent radiologic facility; L Lapatinib; LD Loading dose; MBC Metastatic breast cancer; NR Not reported; ORR Objective response rate; q3w Every-3-week; PFS Progression-free survival; PO Oral; T Trastuzumab; T-DM1 Trastuzumab emtansine; TPC Treatment of physician s choice; X Capecitabine; IV Intravenous |
|||||||||||
|
EMILA (NCT00829166)[37] |
2012 |
RPreviously treated with T and a taxane with centrally confirmed HER2 + un-resectable, locally advanced or MBC |
495 |
T-DM1 (3.6 mg/kg IV, q3w) |
PFS by IRF, OS and safety |
PFS by investigator and ORR |
43.6 |
30.9 |
12.6 |
9.6 |
|
|
496 |
X (1000 mg/m2 PO BID, days 1-14 q3w) + L (1250 mg PO daily) |
30.8 |
25.1 |
6.5 |
6.4 |
||||||
|
2015 |
HER2 + MBC previously treated with a taxane (any setting), and lapatinib plus T (advanced setting) |
404 |
T-DM1 (3.6 mg/kg q3w) |
PFS by investigator and OS |
ORR by investigator and safety |
31 |
22.7 |
NR |
6.2 |
||
|
198 |
TPCa |
9 |
15.8 |
3.3 |
|||||||
|
MARIANNE (NCT01120184)[43] |
2015 |
Recurrent, locally advanced breast cancer or MBC, with no prior chemotherapy for metastatic disease |
365 |
T + D (8 mg/kg LD then 6 mg/kg + 100 or 75 mg/m2 q3w) or T + paclitaxel (4 mg/kg LD then 2 mg/kg + 80 mg/m2 qw) |
PFS by IRF |
OS, PFS by investigator, ORR, safety, patientreported outcomes |
67.9 |
NR |
12.5 |
13.7 |
|
|
367 |
T-DM1 + placebob (3.6 mg/kg + 840 mg LD then 420 mg q3w) |
59.7 |
20.7 |
14.1 |
LC |
||||||
|
363 |
T-DM1 + pertuzumab (3.6 mg/ kg + 840 mg LD then 420 mg q3w) |
64.2 |
21.2 |
15.2 |
|||||||
|
T-DM1 Trastuzumab emtansine; HER2 Human epidermal growth factor receptor 2; RECIST Response evaluation criteria in solid tumor |
|
Inclusion criteria |
|
Progression during or after the most recent treatment for locally advanced or metastatic disease or within 6 months after treatment for early-stage disease, and a centrally confirmed HER2-positive status, assessed by means of immunohistochemical analysis (with 3+ indicating positive status), fluorescence in situ hybridization (with an amplification ratio >2.0 indicating positive status), or both Patients with measurable disease (according to modified RECIST) and those with nonmeasurable disease were included Left ventricular ejection fraction of 50% or more (determined by echocardiography or multiple-gated acquisition scanning) |
|
Eastern Cooperative Oncology Group performance status of 0 (asymptomatic) or 1 (restricted in strenuous activity but ambulatory and able to do light work) |
|
Exclusion criteria |
|
Prior treatment with T-DM1, lapatinib, or capecitabine |
|
Peripheral neuropathy of Grade 3 or higher (according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0) 14 |
|
Symptomatic central nervous system metastases or treatment for these metastases within 2 months before randomization History of symptomatic congestive heart failure or serious cardiac arrhythmia requiring treatment History of myocardial infarction or unstable angina within 6 months before randomization T-DM1 - Trastuzumab emtansine; HER2 - Human epidermal growth factor receptor 2; RECIST - Response evaluation criteria in solid tumor |
|
OS |
Cap + Lap |
T-DM1 |
HR (95% CI) |
P |
Stopping boundary |
|---|---|---|---|---|---|
|
aData cutoff January 2012; bData cutoff July 2012; cData cutoff December 2014. Cap + Lap Capecitabine plus lapatinib; CI Confidence interval; HR Hazard ratio; IA Interim analysis; OS Overall survival; T-DM1 Trastuzumab emtansine; NE Not estimable |
|||||
|
First interim analysis* |
|||||
|
n (percentage OS events) |
129 (26.0) |
94 (19.0) |
0.62 (0.48-0.81) |
0.0005 |
P<0> |
|
Median (months) Second interim analysisb |
23.3 |
NE |
|||
|
n (percentage OS events) |
182 (36.7) |
149 (30.1) |
0.68 (0.55-0.85) |
0.0006 |
P<0> |
|
Median (months) Final analysisc |
25.1 |
30.9 |
|||
|
n (percentage OS events) |
333 (67.1) |
303 (61.2) |
0.75 (0.64-0.88) |
0.0003 |
Boundary met at second |
|
Median (months) |
|||||
|
Sensitivity analysis with crossover patients censoredc |
25.9 |
29.9 |
IA/descriptive only |
||
|
n (percentage OS events) |
278 (56.0) |
303 (61.2) |
0.69 (0.59-0.82) |
<0> |
Descriptive only |
|
Median (months) |
24.6 |
29.9 |
|||

|?Figure.1Trastuzumab emtansine - mechanism of action
References
- iegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9-29
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177-82
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707-12
- bi H, Costa C, Faber SC, Nishtala M, Kotani H, Juric D. et al. PI3K regulates MEK/ERK signaling in breast cancer via the rac-GEF, P-Rex1. Proc Natl Acad Sci U S A 2013; 110: 21124-9
- ahta R. Molecular mechanisms of trastuzumab-based treatment in HER2-overexpressing breast cancer. ISRN Oncol 2012; 2012: 428062
- Kallioniemi OP, Kallioniemi A, Kurisu W, Thor A, Chen LC, Smith HS. et al. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci U S A 1992; 89: 5321-5
- olff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol 2013; 31: 3997-4013
- Brufsky AM. Current approaches and emerging directions in HER2-resistant breast cancer. Breast Cancer (Auckl) 2014; 8: 109-18
- umar G, Badve S. Milestones in the discovery of HER2 proto-oncogene and trastuzumab (herceptin). Connections 2008; 13: 9-14
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783-92
- Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M. et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol 2005; 23: 4265-74
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733-43
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG. et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008; 112: 533-43
- Gamucci T, Moscetti L, Mentuccia L, Pizzuti L, Mauri M, Zampa G. et al. Optimal tolerability and high efficacy of a modified schedule of lapatinib-capecitabine in advanced breast cancer patients. J Cancer Res Clin Oncol 2014; 140: 221-6
- FDA NEWS RELEASE. FDA Approves New Treatment for Late-Stage Breast Cancer. Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm340704.htm. [Last accessed on 2016 Sep 18]
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res 2014; 16: 209
- Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL. et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): An antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 2012; 69: 1229-40
- Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W. et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 2010; 28: 2698-704
- Lewis PhillipsGD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai G. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008; 68: 9280-90
- 0 Peddi PF, Hurvitz SA. Trastuzumab emtansine: The first targeted chemotherapy for treatment of breast cancer. Future Oncol 2013; 9: 319-26
- Bouchard H, Viskov C, Garcia-Echeverria C. Antibody-drug conjugates-A new wave of cancer drugs. Bioorg Med Chem Lett 2014; 24: 5357-63
- Krop I, Winer EP. Trastuzumab emtansine: A novel antibody-drug conjugate for HER2-positive breast cancer. Clin Cancer Res 2014; 20: 15-20
- Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011; 128: 347-56
- Remillard S, Rebhun LI, Howie GA, Kupchan SM. Antimitotic activity of the potent tumor inhibitor maytansine. Science 1975; 189: 1002-5
- Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R. et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther 2010; 9: 2689-99
- Lambert JM, Chari RV. Ado-trastuzumab emtansine (T-DM1): An antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem 2014; 57: 6949-64
- Blum RH, Wittenberg BK, Canellos GP, Mayer RJ, Skarin AT, Henderson IC. et al. A therapeutic trial of maytansine. Cancer Clin Trials 1978; 1: 113-7
- Luo Y, Lacroix JJ, Prabhu S. Ado-trastuzumab emtansine. In: Wang J, Shen WC, Zaro JL. editors Antibody-Drug Conjugates: The 21st Century Magic Bullets for Cancer. New York: New York: Springer; 2015. p. 203-24
- Kovtun YV, Goldmacher VS. Cell killing by antibody-drug conjugates. Cancer Lett 2007; 255: 232-40
- Ritchie M, Tchistiakova L, Scott N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. MAbs 2013; 5: 13-21
- Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K. et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 2006; 66: 4426-33
- Burris 3rd HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011; 29: 398-405
- Lu D, Girish S, Gao Y, Wang B, Yi JH, Guardino E. et al. Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: Clinical implications of the effect of covariates. Cancer Chemother Pharmacol 2014; 74: 399-410
- Li C, Agarwal P, Dent S, Goncalves A, Yi JH, Strasak A. et al. Phase 1 Study of Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer Patients with Normal or Reduced Hepatic Function [abstract]. In: Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium, 9-13 December, 2014; San Antonio, TX, Philadelphia (PA): AACR; Cancer Res 2015;75 9 Suppl: Abstract nr p. 4-15-9
- Bajaj N, Shaaban H, Guron G, Maroules M. The role of trastuzumab emtansine as a novel-targeted therapy for HER2+breast cancer: A systematic review. Clin Cancer Invest J 2013; 2: 275-80
- Krop IE, LoRusso P, Miller KD, Modi S, Yardley D, Rodriguez G. et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol 2012; 30: 3234-41
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012; 367: 1783-91
- Di ras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J. et al. Trastuzumab Emtansine Improves Overall Survival Versus Capecitabine Plus Lapatinib in Patients with Previously Treated HER2-Positive Advanced Breast Cancer: Final Results from the Phase 3 EMILIA study. Presented at: 38th Annual San Antonio Breast Cancer Symposium, 8-12 December, 2015; San Antonio, TX, USA; Poster # p. 4-14-1
- European Medicines Agency. Kadcyla (Trastuzumab Emtansine): Summary of Product Characteristics; 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/002389/WC500158593.pdf. [Last updated on 2017 Jan 13; Last accessed on 2017 Feb 09].
- Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M. et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann Oncol 2015; 26: 113-9
- Krop IE, Kim SB, Gonz lez-Mart n A, LoRusso PM, Ferrero JM, Smitt M. et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 689-99
- Wildiers H, Kim S-B, Gonzalez-Martin A, LoRusso PM, Ferrero J-M, Yu R. et al. Trastuzumab Emtansine Improves Overall Survival Versus Treatment of Physician's Choice in Patients with Previously Treated HER2-Positive Metastatic Breast Cancer: Final Overall Survival Results from the Phase 3 TH3RES A Study. Presented at: 38th Annual San Antonio Breast Cancer Symposium, 8-12 December, 2015; San Antonio, TX, USA. Oral Session #S5-5
- Ellis P, Barrios CH, Eiermann W, Toi M, Young-Hyuck IM, Conte P. et al. Phase III, Randomized Study of Trastuzumab Emtansine (TDM1) Pertuzumab (P) vs. Trastuzumab + Taxane (HT) for Firstline Treatment of HER2 Positive MBC: Primary Results from the MARIANNE Study. Presented at: 51st Annual Meeting of American Society Of Clinical Oncology, June 3-7, 2015; Chicago, IL, USA. Oral Abstract # 507
- Kadcyla (Trastuzumab Emtansine for Injection) [Product Information India]. Roche Products (India) Pvt. Ltd. February, 2015, Ver. 3.0.
- Yardley DA, Krop IE, LoRusso PM, Mayer M, Barnett B, Yoo B. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer previously treated with chemotherapy and 2 or more HER2-targeted agents: Results from the T-PAS expanded access study. Cancer J 2015; 21: 357-64
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer (Ver. 2. 2016). Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Last accessed on 2017 Feb 09]
- Hanf V, Sch tz F, Liedtke C, Thill M. AGO Breast Committee. AGO recommendations for the diagnosis and treatment of patients with advanced and metastatic breast cancer: Update 2014. Breast Care (Basel 2014; 9: 202-9
- Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andr F. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014; 23: 489-502
- Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE. et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2014; 32: 2078-99
- A Study of Trastuzumab Emtansine versus Trastuzumab as Adjuvant Therapy in Patients With HER2-Positive Breast Cancer Who Have Residual Tumor in the Breast or Axillary Lymph Nodes Following Preoperative Therapy (KATHERINE). Available from: https://www.clinicaltrials.gov/ct2/show/NCT01772472 term=NCT01772472&rank=1. [Last accessed on 2017 Feb 09]
- A Study of Kadcyla (Trastuzumab Emtansine) Plus Perjeta (Pertuzumab) Following Anthracyclines in Comparison With Herceptin (Trastuzumab) Plus Perjeta and a Taxane Following Anthracyclines as Adjuvant Therapy in Patients With Operable HER2 Positive Primary Breast Cancer. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01966471 term=NCT01966471&rank=1. [Last accessed on 2017 Feb 09].


 PDF
PDF  Views
Views  Share
Share

