Therapeutic approach beyond conventional temozolomide for newly diagnosed glioblastoma: Review of the present evidence and future direction
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(04): 229-237
DOI: DOI: 10.4103/0971-5851.171543
Abstract
Glioblastoma multiforme (GBM) is the most aggressive form of primary brain tumor. Maximal safe surgical resection followed by adjuvant partial brain radiation with concurrent and adjuvant temozolomide (TMZ) (oral alkylating agent) is the standard of care. Five years survival in TMZ treated patient reaches 9.8%. We aimed to summarize the changes in the management of GBM beyond conventional temozolomide based adjuvant treatment. We searched the PUBMED with the following key words: Glioblastoma, phase III trial, Phase II trial, adjuvant treatment in GBM. Clinical research has found a wide range of molecular aberrations in GBM and attempts are being made to further improve survival with the addition of different classes of drugs. Angiogenesis inhibitors, oncolytic vaccines, dose dense TMZ, and anti-epidermal growth factor receptor monoclonal antibody in phase III trials have failed to improve survival. Recent studies have also shown that the management strategies might be different and needs to be customized as per the age of patients such as pediatric and elderly patients. In addition, treatments should be personalized depending on the molecular aberrations. We reviewed all published phase III trials for newly diagnosed GBM as well as also looked into possible future directions in this review. Limited progress has happed beyond conventional TMZ in the adjuvant treatment of GBM. Newer insights are emerging about treatment intensification and introduction of newer molecular targeted drugs with more information about molecular aberrations.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Glioblastoma multiforme (GBM) is the most aggressive form of primary brain tumor. Maximal safe surgical resection followed by adjuvant partial brain radiation with concurrent and adjuvant temozolomide (TMZ) (oral alkylating agent) is the standard of care. Five years survival in TMZ treated patient reaches 9.8%. We aimed to summarize the changes in the management of GBM beyond conventional temozolomide based adjuvant treatment. We searched the PUBMED with the following key words: Glioblastoma, phase III trial, Phase II trial, adjuvant treatment in GBM. Clinical research has found a wide range of molecular aberrations in GBM and attempts are being made to further improve survival with the addition of different classes of drugs. Angiogenesis inhibitors, oncolytic vaccines, dose dense TMZ, and anti-epidermal growth factor receptor monoclonal antibody in phase III trials have failed to improve survival. Recent studies have also shown that the management strategies might be different and needs to be customized as per the age of patients such as pediatric and elderly patients. In addition, treatments should be personalized depending on the molecular aberrations. We reviewed all published phase III trials for newly diagnosed GBM as well as also looked into possible future directions in this review. Limited progress has happed beyond conventional TMZ in the adjuvant treatment of GBM. Newer insights are emerging about treatment intensification and introduction of newer molecular targeted drugs with more information about molecular aberrations.
INTRODUCTION
Glioblastoma multiforme (GBM) is the most aggressive type of brain tumor arising from glial cells accounting for 52% of all parenchymal brain tumor cases and 20% of all intracranial tumors[1] GBM has a pronounced mitotic activity, substantial tendency toward neoangiogenesis (microvascular proliferation), necrosis, and proliferative rates 3-5 times higher than anaplastic astrocytoma.[1] Because of their intrinsic infiltrative nature, GBM has a highly aggressive malignant clinical course. The adjuvant chemoradiotherapy (RT) with temozolomide (TMZ) after maximal safe resection remains the standard of care. We intend to discuss the present status as well as a peep into the future directions for the management of this disease with a grim prognosis.
THE TEMOZOLOMIDE ERA OF CONVENTIONAL AND ALTERED DOSE TEMOZOLOMIDE
Conventional dose of temozolomide with concurrent radiation
Stupp et al. in a landmark phase III trial compared adjuvant radiation alone with or without TMZ (an alkylating agent) for newly diagnosed GBM patients. At a median follow-up of 28 months, the median overall survival (OS) was reported to be 14.6 months with RT plus TMZ group and 12.1 months with RT alone group. The 2-year survival rate was 26.5% with RT plus TMZ and 10.4% with RT alone with an acceptable 7% grade 3/4 hematologic toxicity in the concomitant treatment arm.[2] Updated results reported 5 years OS of 9.8% with TMZ, versus 1.9% with RT alone.[3] This trial established a new paradigm in the treatment of GBM and TMZ concurrent with radiation and maintenance for six cycles became the standard of care for newly diagnosed cases of GBM. Subsequently, Hegi et al. established epigenetic silencing of the O6-methylguanine-DNA methyltransferase (MGMT) DNA-repair gene by promoter methylation and this has been associated with longer survival in patients with GBM, who receive alkylating agents. It was interesting to see a survival benefit even in the MGMT unmethylated tumors when treated with TMZ compared with MGMT unmethylated tumors treated with radiation alone albeit lesser than the methylated patients. Median survival was 21.7 months compared to 15.3 months for MGMT methylated patients when treated with radiation with or without TMZ, respectively.[4] This may be contributed by the possible radiation sensitizing effect of TMZ. Hence, it remained an important clinical question whether the concurrent or the maintenance TMZ played the crucial role in improving disease control. However, it will be unethical to conduct a trial without the maintenance TMZ now. Julka et al. reported similar survival and toxicity patterns in a large series from India reflecting the beneficial effects of TMZ along with radiation in Asian patients as well.[5]
Dose dense temozolomide with concurrent radiation
It was also hypothesized that continuous daily administration of TMZ is more effective than a single dose. Hence, clinical trials with TMZ explored a wide range of dosing schedules aiming maximum MGMT depletion in tumor cells. These regimens increased the exposure of TMZ by 1.5-2 folds compared to the conventional 5-day schedule of TMZ.[4] This concept of persistent MGMT depletion was the basis of the RTOG 0525 trial which randomly assigned 833 patients with newly diagnosed GBM to receive standard radiation and six cycles maintenance TMZ (150-200 mg/m2 D1-D5 q 4 weekly) or dose-dense TMZ (75-100 mg/m2 D1-D21 q 4 weekly) for 12 cycles.[6] However, this trial failed to improve either median OS (16.6 vs. 14.9 months; hazard ratio [HR], 1.03; P = 0.63) or median progression-free survival (PFS) (5.5 vs. 6.7 months; HR, 0.87; P = 0.06). Efficacy was not different even by the MGMT methylation status; however, grade 3 or higher toxicity was significantly increased in the dose-dense arm.
Radiation or temozolomide monotherapy for elderly glioblastoma multiforme
The adjuvant trials of concurrent and adjuvant TMZ excluded patients who were elderly and frail. A new set of phase III trials was conducted to formulate the optimum therapy for these patients. The German NOA-8 trial randomly assigned 412 patients with age 65 years or more or with a performance status of 60 or higher to receive 100 mg/m2 TMZ, given on days 1-7 of 1 week on, 1 week off cycles, or RT of 60 Gy, delivered over 6-7 weeks in 30 fractions.[7] The primary endpoint was OS. This was a noninferiority trial with a 25% margin. Median OS (8.6 months vs. 9.6 months, p = 0·033), and event-free survival (EFS) (3.3 months vs. 4.7, P noninferiority = 0·043) were not significantly different between the TMZ and RT groups. The trial also found MGMT methylation as an important biomarker determining the survival outcome as MGMT methylated patients had a better EFS (8.4 months vs. 4.6 months) when treated with TMZ whereas unmethylated patients had better EFS (3.3 months vs. 4.6 months) when treated with radiation. Grade 3 or higher toxicity was more commonly reported in the TMZ treated patients. A similar trial (NORDIC) evaluated the treatment outcome in newly diagnosed patients of GBM with age more than 70 years or older.[8] The trial randomly assigned 291 patients to receive either TMZ (200 mg/m2 on days 1-5 of every 28 days for up to six cycles), hypofractionated RT (34 Gy administered in 3.4 Gy/fractions over 2 weeks), or standard RT (60 Gy in 30 fractions over 6 weeks). OS was not different between patients treated with hypofractionated radiation or TMZ (7.4 vs. 8.4 months, P = 0.12). However, OS was significantly better in hypofractionated radiation or TMZ arm compared to the standard radiation alone arm. MGMT methylated patients when treated with TMZ had improved survival (9.7 months vs. 6.8 months) but survival was not different for both MGMT methylated and unmethylated tumors when treated RT. Neutropenia (12% vs. 0%), thrombocytopenia (21% vs. 0%), and Infection/fever (19% vs.7%) was found more frequently in the TMZ treated patients compared with radiation treated patients. The point should be made that the NORDIC trial deliberately did not collect data on the treatment at progression. Patients in the TMZ arm after progression may have received radiation and vice versa which may have masked the results. Hence, hypofractionated radiation or TMZ appears equally efficacious; however, toxicity remains significantly higher in the TMZ group which may lead to poor treatment compliance in these elderly/frail patients. Hence, in MGMT methylated subset, TMZ alone may be used albeit toxicity may limit the true benefit. Whereas MGMT unmethylated patients should be considered for hypofractionated radiation only.
ANGIOGENESIS INHIBITOR IN ADJUVANT TREATMENT
Bevacizumab
Extensive angiogenesis and marked microvascular proliferation in GBM paved for evaluation of bevacizumab, a humanized monoclonal antibody targeted against vascular endothelial growth factor-A (VEGF-A). Clinical trials in recurrent GBM showed dramatic response when treated with bevacizumab and this paved way for newer trials for newly diagnosed patients with GBM. Antiangiogenic treatment is generally well tolerated. However, common adverse effects include hypertension and proteinuria. A Smaller number of cases experience serious adverse effects, such as thromboembolic disease and hemorrhage. Two landmark phase III trials evaluated this hypothesis. Gilbert et al. in a phase III trial randomized 978 patients to receive standard radiation and TMZ with or without bevacizumab.[9] Bevacizumab (or placebo) was used 10 mg/kg every 2 weeks, starting at week 4 of RT, until disease progression, severe treatment-related toxicity, or completion of adjuvant therapy (maximum number of doses, 24 over 12 cycles). At a median follow-up of 20.5 months, OS between the bevacizumab group and the placebo group was not different (median, 15.7 vs. 16.1 months). However, the PFS was significantly improved in the experimental arm (10.7 vs. 7.3 months, P = 0.007). The class side effects: Hypertension, thromboembolic events, intestinal perforation, and neutropenia were more common in the bevacizumab treated group. Notably, patients in the experimental arm experienced an increased symptom burden, a worse quality of life (QOL), and a decline in neurocognitive function. The other trial (AVAglio) randomly assigned 458 patients with newly diagnosed GBM to receive radiation and TMZ with or without bevacizumab.[10] Bevacizumab in this trial was initiated after 4 weeks of surgery at 10 mg/kg every 2 weeks followed by a similar dose of bevacizumab with maintenance TMZ for six cycles. Finally, bevacizumab monotherapy (15 mg/kg) or placebo was continued every 3 weeks until the disease progressed or unacceptable toxic effects happened. Median PFS was 10.6 months in the bevacizumab group compared with 6.2 months in the placebo group (P < 0.001). However, this trial reported superior PFS in addition to maintained health-related QOL, performance status, and lower glucocorticoid requirement. Grade 3 or higher adverse events were more common in patients in the bevacizumab group than in the placebo group (66.8% vs. 51.3%). Sandmann et al. recently reported the results of biomarker analysis of the patients treated in the AVAglio trial. A total of 349 patients were profiled for gene expression and isocitrate dehydrogenase 1 (IDH1) mutation status and classified into previously identified molecular subtypes. The analysis revealed significant survival advantage when treated with bevacizumab compared to placebo for proneural IDH1 wild-type tumors (17.1 vs. 12.8 months). Both the mesenchymal and proneural tumors had a PFS benefit from bevacizumab, but it was translated to OS for proneural type only.[11] This selective benefit in IDH wild-type proneural GBM may be because of intrinsic resistance of mesenchymal tumors towards anti-VEGF therapy. This retrospective analysis showed 4.3 months OS benefit for patients in IDH wild-type proneural GBM, which was found to be the GBM with worst prognosis and hence these results may appear appealing and potentially practice changing. In a recent economic evaluation of bevacizumab for the first-line treatment of newly diagnosed GBM, Kovic and Xie reported very limited effectiveness.[12] The authors concluded that bevacizumab is more cost effective for recurrent tumors as a salvage regimen but not in the first line. However, this analysis was published before the finding of Sandmann et al.[11] and in that context bevacizumab may be considered even in the first line for the IDH wild-type proneural GBM. Taphoorn et al. evaluated QOL with the European Organization for Research and Treatment of Cancer QOL Questionnaires C30 and BN20 at each tumor assessment in patients of the AVAglio study.[13] The authors reported statistically longer (P < 0.001) deterioration-free survival with the addition of bevacizumab to RT/TMZ. However, the QOL report from the RTOG 0825 trial is not in agreement to the AVAglio trial. These conflicting results of the two large trials points towards the doubtful benefit of adding bevacizumab in the adjuvant therapy protocol.
Novel antiangiogenic therapy
Integrins are a family of cell-cell and cell-extracellular matrix adhesion molecules. These integrins are involved in various cellular processes including cell survival, proliferation, migration, invasion, and angiogenesis. In particular, αvβ3 and αvβ5 integrins are considered key mediators of crosstalk between tumor cells and the brain microenvironment in GBM and are over expressed on tumor cells and vasculature.[14] Cilengitide is a selective inhibitor of αvβ3 and αvβ5 integrins and showed promising results in phase I/II studies for recurrent or newly diagnosed GBM. Stupp et al. in a phase III trial randomly assigned 545 newly diagnosed MGMT methylated GBM cases to receive either standard chemo-RT or chemo-RT with cilengitide (standard dose of 2000 mg intravenously twice weekly on days 1 and 4, beginning 1 week before starting TMZ and RT).[15] Cilengitide was continued for up to 18 months or until disease progression or unacceptable toxic effects. The primary endpoint was OS. Secondary endpoints were PFS and safety. Median OS was identical 26.3 months in both the arms. Even 2 years survival was 56% in both the study arms. Median PFS was 13.5 months in the experimental arm and 10.7 months in the standard arm but could not reach statistical significance. The trial also reported a similar rate of treatment-emergent adverse events (64% vs. 61%). The failure of this trial to improve survival in the back ground of promising phase I/II trial results warns about the aggressive nature of GBM, which is rich in diverse molecular aberration and not amenable to single pronged approach. In this regard, extensive preclinical modeling and next generation sequencing may help to derive future trial design.
ANTI-EPIDERMAL GROWTH FACTOR RECEPTOR MONOCLONAL ANTIBODY
More than 60% of GBM overexpress epidermal growth factor receptor (EGFR). In about half of these, overexpression is the result of a mutant form of the receptor, EGFR-vIII, which has a constitutively active kinase domain. EGFR has long been implicated in the gliomagenesis.[16] Hence, anti-EGFR therapy in the form of anti-EGFR monoclonal antibody and small molecule tyrosine kinase inhibitors have been evaluated for the treatment of GBM but with limited or no benefit. Subsequently, it was realized that the constitutively active form of EGFR the EGFR-vIII is responsible for the limited effectiveness of this anti-EGER therapy. Nimotuzumab is a monoclonal antibody against EGFR without intrinsic stimulating activity. Because of lesser affinity, this drug binds more specifically to EGFR overexpressing cells. The encouraging results of phase II trials paved the way for phase III trial with nimotuzumab in newly diagnosed GBM.[17] The trial randomly assigned 149 patients with newly diagnosed GBM to receive either intravenous nimotuzumab 400 mg weekly added to standard radiochemotherapy followed by 400 mg biweekly after 12 weeks or standard radiochemotherapy only.[18] The primary endpoint was progression status after 52 weeks and PFS. OS, toxicity, and QOL were secondary endpoints. Median PFS was 7.7 months in the experimental arm compared to 5.8 months in the standard arm with a nonsignificant P value. 12 months PFS rate was 22% versus 18% in the experimental and standard arm, respectively. Median OS for the experimental arm was 22.3 months versus 19.6 months in the standard arm and difference was not statistically significant. Interestingly, EGFR amplification did not correlate with clinical efficacy of nimotuzumab.
ONCOLYTIC VIRUS IN THE ADJUVANT MANAGEMENT OF GLIOBLASTOMA MULTIFORME
In the recent years, great enthusiasm has been witnessed for gene therapy as a therapeutic approach for newly diagnosed or recurrent GBM. These gene therapies mainly act as local consolidation akin to carmustine wafers, which are the only approved local intracavity therapy. Using an adenoviral vector with a high titer, adenovirus-mediated HSV-tk gene therapy administered locally, intraoperatively, and in conjunction with subsequent intravenous ganciclovir has been developed for the treatment of operable high-grade glioma.[19] The active agent, sitimagene ceradenovec (Ark Therapeutics Ltd., London, UK) is a first-generation replication-deficient adenovirus (serotype 5 with E1 and partial E3 deletions) containing the cDNA for HSV-tk. Transgene-expressing cells produce thymidine kinase, which phosphorylates ganciclovir to ganciclovir triphosphate, a cytotoxic nucleotide analog that selectively kills dividing cells by being incorporated into DNA and leads to apoptosis both in transduced cells and adjacent dividing cells through a so-called bystander effect. This process spares normal neurons because they do not proliferate and are therefore not susceptible to the toxic effects of ganciclovir metabolites. In a recent phase III trial, Westphal et al. assessed the efficacy and safety of a locally applied adenovirus-mediated gene therapy with a prodrug converting enzyme (herpes-simplex-virus thymidine kinase; sitimagene ceradenovec) followed by intravenous ganciclovir in patients with newly diagnosed resectable GBM.[20] The study randomly assigned 250 patients to receive either surgical resection of the tumor and intraoperative perilesional injection of sitimagene ceradenovec (1 × 1012 viral particles) followed by ganciclovir (postoperatively, 5 mg/kg intravenously twice a day) in addition to standard care or resection and standard care alone. However, TMZ was not available in all participating countries and was delivered at physician's discretion only. Median time to death or re-intervention was longer in the experimental group (308 days, 95% confidence interval [CI]: 283-373) than in the control group (268 days, 210-313; HR: 1.53, 95% CI: 1.13-2.07; P = 0·006). More patients in the experimental group had one or more treatment-related adverse events those in the control group (88 [71%] vs. 51 [43%]). The most common grade 3-4 adverse events were hemiparesis (eight in the experimental group vs. three in the control group) and aphasia (six vs. two).
HYPOFRACTIONATED RADIATION WITH CONCURRENT CHEMOTHERAPY
Even after adjuvant treatment of a newly diagnosed case of GBM, local recurrence happens to be the most common cause of disease recurrence. In an earlier trial, dose escalations with conventional fractionation or hyperfractionation attempted to improve disease control. However, these trials failed to achieve any benefit but increased the rate of radionecrosis. In the recent time, the analysis of the pattern of failure revealed that 85-90% recurrences occur inside the radiation field or high dose area indicating a possible role of dose escalation.[21] However, dose escalation in conventional fractionation losses its edge because of increased overall treatment time. The loss of therapeutic window after the 4th week of radiation because of accelerated repopulation has emerged as a potential area of research. Efforts are being made to complete the treatment in < 6 weeks and at the same time without total dose reduction. Hence, hypofractionated radiation alone or in combination with TMZ or other chemotherapy drugs is being evaluated in phase I/II trials for newly diagnosed GBM.[22,23,24,25,26] This approach enables an increase in the biological equivalent dose and a possible tumor ablative action in addition to DNA damaging property of conventional radiation. Panet-Raymond et al. delivered a dose of 40 and 60 Gy in 20 fractions to the low and high-risk clinical target volume. The authors reported comparable outcome and toxicity results compared to the standard arm.[26] Subsequently, few other publications have attempted to compare the hypofractionated RT and achieved encouraging results [Table 1]. However, there is no properly designed comparison between conventional and hypofractionated RT. The results of hypofractionated radiation are summarized in Table 2.
Table 1
Summarizes different hypofractionated radiation with concurrent chemotherapy study for glioblastoma multiforme
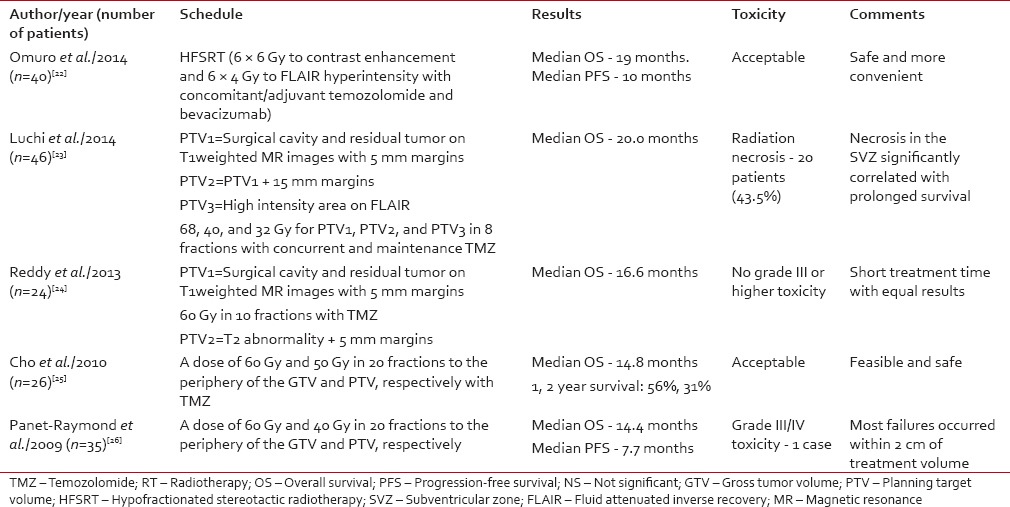
Table 2
Summarizes ongoing phase III trials with novel agents

NEOADJUVANT CHEMOTHERAPY FOLLOWED BY RADIATION AND TEMOZOLOMIDE
Nimustine (ACNU) or carmustine (BCNU) and lomustine (CCNU) have long been used for the treatment of glioma even before the advent of TMZ. Investigators attempted to combine the cytotoxic effects of dual alkylating agent for the newly diagnosed GBM to intensify the treatment.[27,28] Kim et al. randomly assigned patients to receive RT and six cycles of adjuvant TMZ with or without two cycles of ACNU/cisplatin neoadjuvant chemotherapy.[27] The primary endpoint was OS. The study was terminated after an interim analysis reported a high frequency of toxicity profiles. By that time, 82 patients were randomized. Median OS was 28.4 months in the treatment group and 18.9 months in the control group (P = 0.2). The 2-year survival rate and progression-free survival time were 50.9% and 6.6 months in the treatment group and 27.8% and 5.1 months in the control group.
UNCONVENTIONAL THERAPY (NONCHEMOTHERAPY APPROACH)
In the recent years, efforts have also been made to improve outcome with nonconventions therapy like NovoTTF. The NovoTTF-100A system (Novocure Ltd., Haifa, Israel) is a portable device with the capacity to deliver low intensity, intermediate frequency, alternating electric fields (Tumor Treating Fields) with noninvasive, and disposable transducer arrays. These fields lead to misalignment of the microtubule subunits in the mitotic spindle during the metaphase to anaphase transition and dielectrophoretic movement of intracellular micromolecules and organelle during telophase and thereby interfering with cell division and cell death.[29] Stupp et al. in a phase III study compared this NovoTTF with physician's choice chemotherapy for recurrent GBM. However, this therapy failed to improve survival in recurrent GBM.[30] The median survival was approximately 6 months in both the arm with 1-year survival of 20% in both arms. However, a post-hoc analysis of the trial[31] reported significantly improved survival for NovoTTF treated patients with a maximal monthly compliance rate ≥75% (≥18 h daily) versus those with a < 75% compliance rate (7.7 vs. 4.5 months; P = 0.042).[30] Such novel treatment approach merits careful consideration in future trials in properly selected GBM patients.
TREATMENT FOR PEDIATRIC GLIOBLASTOMA MULTIFORME
Pediatric GBM is always considered a distinct entity of GBM as these tumors are molecularly distinct from the adult counterpart. In pediatric patients, MGMT gene promoter methylation has been reported in 50% patients and p53 protein expression in 60% of cases.[32] Genome-wide small noncoding RNA profiling of pediatric high-grade gliomas revealed deregulation of different miRNAs. This study reported two distinct classes of pediatric high-grade gliomas and reported a better OS for tumors with down regulated 14q32 cluster.[33] Pathak et al. recently evaluated global histone code (H3K-4/9/27/36) trimethylation pattern in H3F3A-ATRX mutants and wild type. The authors reported H3F3A-ATRX mutation in 66.7% of pediatric GBMs. Similarly, K27M and G34R-H3F3A mutations were found in 37% and 14.8% patients respectively.[34] Jha et al. evaluated genome-wide methylation profiling in pediatric GBM cases. Pediatric GBM was characterized by 94 hypermethylated and 1206 hypomethylated cytosine-phosphate-guanine islands, with 3 distinct clusters.[35] This indicates toward the existence of epigenetic subgroups within pediatric GBM. These studies hint toward considerably heterogeneity even in the small cohort of pediatric GBM. Earlier radiation was advocated to be the only adjuvant therapy for these tumors. However, recent series of pediatric GBM has reported encouraging results and manageable toxicity when treated with concurrent radiation and TMZ followed by maintenance TMZ.[36] Another series of 66 patients reported recently,[37] found the median survival to be 15 months when treated with the same schedule. In addition, MGMT methylation and p53 over expression was not shown to impact OS.
NOVEL AGENTS IN PHASE II TRIALS
Selective serine/threonine kinase inhibitor of protein kinase C: Enzastaurin
Enzastaurin is a selective serine/threonine kinase inhibitor of protein kinase C (PKC). These PKC enzymes are responsible for tumor growth, proliferation, and apoptosis. Enzastaurin disrupts phosphotransferase activity of PKC isoforms via an interaction at the ATP binding site. Inhibition of this pathway by enzastaurin blocks tumor angiogenesis and growth.[38] In a phase II trial, Butowski et al. subjected 66 patients with newly diagnosed GBM to receive standard radiochemotherapy with TMZ with enzastaurin given once daily during RT and in the adjuvant period at 250 mg/day without dose modifications. The primary endpoint was OS. Median OS was 17.1 months, slightly more favorable than the standard treatment and median PFS was 9 months. The most common grade 3/4 adverse event was lymphopenia noted in 39.4% patients.[39] In another trial, enzastaurin was delivered before and concomitant with radiation therapy, followed by enzastaurin maintenance therapy in newly diagnosed GBM patients without MGMT promoter hyper-methylation. The primary endpoint was PFS at 6 months of at least 55%. However, the trial reported 53.6% PFS at 6 months (95% CI: 39.865.6) and median OS of 15.0 months (95% CI: 11.917.9) for all patients.[40]
Vascular endothelial growth factor and basic fibroblast growth factor inhibitor: Thalidomide
Overexpression of pro-angiogenic molecules such as VEGF and basic fibroblast growth factor (bFGF) has been considered typical of high-grade glioma.[41] Thalidomide (N-phthalylglutamic acid imide) was found to inhibit VEGF and bFGF in an animal model. RTOG 9806 was a single arm study to evaluate safety and efficacy of daily thalidomide with radiation therapy in patients with newly diagnosed GBM. Thalidomide was delivered in 200 mg of the daily dose before sleep starting with the first fraction of radiation. The dose was increased every 1-2 weeks by 100 mg-200 mg to 1200 mg as tolerated and continued as 8 weekly cycles until disease progression. The primary endpoint was OS with secondary endpoints of PFS and toxicity. The median survival time was 10 months not different from the historical cohort with increased toxicity in the form of venous thrombosis, fatigue, skin reactions, encephalopathy, and neuropathy.[42]
Farnesyltransferase inhibitor: Tipifarnib
FGF-2 has been reported to controls radioresistance through RhoB, whose farnesylated form modulates radioresistance in GBM. Ducassou et al. treated 27 patients of newly diagnosed GBM with tipifarnib in continuous administration with RT starting 1 week before the initiation of radiation and TMZ was delivered at progression. Median OS was 80.3 weeks and median time to progression was 18.1 weeks. The study found FGF receptor-1 over-expression and avb3 as a negative prognostic factor.[43]
Epidermal growth factor receptor tyrosine kinase inhibitor: Gefitinib
Gefitinib, an EGFR tyrosine kinase inhibitor was used along with radiation for newly diagnosed GBM patients in RTOG 0211 trial. Treatment consisted of daily oral gefitinib (500 mg) started at the time of radiation and continued after radiation for 18 months or until progression. The trial reported median survival of 11.5 months only.[44]
Mammalian target of rapamycin inhibitors: Everolimus
Loss of PTEN function in GBM leads to increased AKT activity which activates the mammalian target of rapamycin (mTOR) through TSC1/2 and Rheb. mTOR inhibitors reduce tumor cell proliferation and tumor growth. Baselga et al.[45] in a landmark phase III trial (which randomized advanced hormone receptor positive breast cancers to everolimus plus exemestane versus exemestane plus placebo) showed a significant improvement in median PFS with the addition of everolimus (10.6 vs. 4.1 months). Drawing from the success of mTOR inhibitors in breast cancers, Hainsworth et al. evaluated the impact of VEGF inhibitor with everolimus (an mTOR inhibitor) when combined with standard radiation therapy and TMZ for newly diagnosed GBM. Median PFS was 11.3 months, and median OS was 13.9 months. Patients experienced the class side effects of mTOR inhibitors as well as VEGF inhibitors without much improvement in outcome.[46]
FUTURE DIRECTION
GBM is one of the most active areas of research. Significant efforts are being made to look beyond basic morphology. Cancer Genome Atlas Network recently identified a large number of genomic abnormalities in GBM. In a pioneering work Verhaak et al. established four different classes of GBM viz., proneural, neural, classical and mesenchymal subtypes with distinct molecular aberration and clinical behavior.[47] Recently, the retrospective analysis of the AVAglio trial reported 4.3 months incremental survival in this proneural subgroup.[11] Hence, patient selection and personalization of treatment should be done with more appropriateness in future. However, the complexity of performing these molecular assays in the lab appears to be labor and cost intensive and may limit routine use. In this context, a simplified model incorporating MGMT methylation, human telomerase (TERT) methylation, and IDH mutation may be formulated to dictate the optimum treatment. Treatment personalization may further be refined with the incorporation of these molecular factors along with patient factors like age, performance status, etc., (molecular-clinical profiling). A Large number of newer drugs and virus based therapy are being evaluated in different phase III and phase II trials as well. The ongoing Phase IIII trials have been summarized in Table 2. The subventricular zone (SVZ) forms the lining the lateral ventricles and represents the origin of neural and some cancer stem cells. Gupta et al.[48] reported on dose volume parameters of SVZ in 40 patients of adult GBM. Dose to the ipsilateral SVZ dose was found to be an independent predictor of survival in multivariate analysis in this study. Although a novel finding, this requires further validation in a prospective study.
CONCLUSION
Based on the available literature, maximal safe surgical resection followed by adjuvant radiation with concurrent TMZ and maintenance six cycles TMZ remains the standard for adult patients with newly diagnosed GBM. Bevacizumab may be added for IDH wild type proneural patients. Hypofractionated radiation or TMZ alone has emerged as treatment options for elderly patients or patients with poor performance status. Although pediatric GBM shows considerable variation from the adult counterpart, recent series reported equivalent outcome when treated with an adult protocol of radiation and TMZ. Hypofractionated radiation is emerging as safe and equally effective treatment option for adult GBM. The oncolytic virus has emerged as a promising approach. Molecular profiling and newer targeted therapy should be explored for improving outcome. A stepwise approach is suggested based on available evidence [Figure 1].
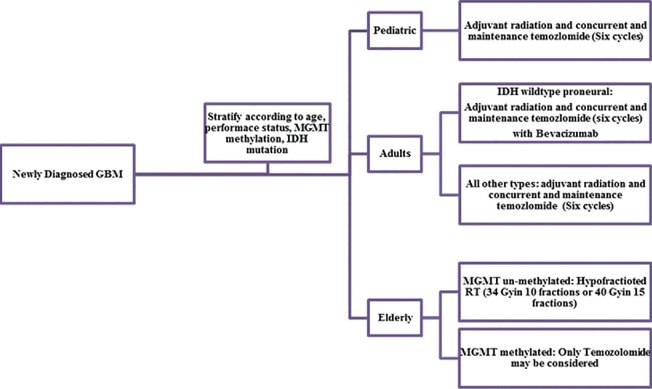
| Fig. 1 Flow chart depicting therapeutic options for newly diagnosed glioblastoma multiforme
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.

| Fig. 1 Flow chart depicting therapeutic options for newly diagnosed glioblastoma multiforme


 PDF
PDF  Views
Views  Share
Share

