The treatment of chronic myeloid leukemia, data from Gujarat Cancer and Research Institute, Ahmedabad
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 189-192
DOI: DOI: 10.4103/0971-5851.123729
Abstract
Gujarat Cancer and Research Institute, Ahmedabad presented data of total 840 patients, out of which 775 (90%) were in chronic phase. Complete hematological response (CHR) was seen in 96% of patients and median time to achieve (CHR) was 2 months. Complete cytogenetic response was seen in 36%.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Gujarat Cancer and Research Institute, Ahmedabad presented data of total 840 patients, out of which 775 (90%) were in chronic phase. Complete hematological response (CHR) was seen in 96% of patients and median time to achieve (CHR) was 2 months. Complete cytogenetic response was seen in 36%.
INTRODUCTION
Chronic myelogenous leukemia was recognized as a distinct entity in the mid-1800s. The modern history of chronic myeloid leukemia (CML) was initiated by Nowell and Hungerford in 1960. They used newly developed techniques to detect a small chromosome in metaphase preparations of marrow cells from CML patients.[1]
CML accounts for 15% of cases of leukemia in the United States. There is a slight male preponderance (male-to-female ratio 1.6:1). Its annual incidence is about 1.5 cases per 100,000 individuals. This incidence has not changed over in the past few decades and it increases with age. The median age at diagnosis is 55-60 years; it is uncommon in children and adolescents; only 2.7% of CML cases are younger than 20 years.
Incidence remains almost constant all over the world. CML is the most common adult leukemia in India.[2,3]
In the 1990s, the recommended approach to manage a newly diagnosed patient with CML-chronic phase (CP) was allogeneic stem cell transplantation (allo-SCT), especially if the patient was relatively young and had suitable donor; for other patients’ interferon-alpha with or without cytarabine was recommended.
From 2000 onward imatinib at 400 mg daily became the preferred initial treatment[4] and this practice received substantial support from the interim results of the immediate risk-stratification improves survival (IRIS) study published in 2003;[5] the 7-year update for patients who received imatinib as first-line treatment showed an actuarial overall survival of 86% and also showed that responding patients whose disease had not progressed in any way in their first 3 years of the study were extremely unlikely to relapse at a later stage and also unlikely to suffer from any late onset side effects. However, the 7-year update also showed that only 57% of the original patient cohort was still in continuing complete cytogenetic response (CCyR) taking imatinib on study according to the original protocol.[6]
A case can be made for offering initial treatment by transplantation for the rare patient with a genetically identical twin since the risk of transplant-related mortality is extremely low with syngeneic donors. Until recently some investigators recommended an initial allograft for younger patients with a high probability of surviving the transplant based on the European scoring system,[7] but even this approach has now fallen from favor.
PATIENTS AND METHODS
Retrospective analysis of cases of CML enrolled in Glivec International Patient Assistance Program at our center from February 2002 to July 2009 was done.
Assessment of new patient of CML at our center includes the following:
- History and physical examination, complete blood count with differential and platelet count and chemistries.
- Bone marrow aspiration and biopsy if aspiration dry.
- Testing for the presence of the Philadelphia (Ph) chromosome by cytogenetic analysis and for the presence of the BCR-ABL fusion gene by fluorescence in situ hybridization (FISH). Reverse-transcription polymerase chain reaction (PCR) not available at Gujarat Cancer and Research Institute (GCRI).
- We are following World Health Organization criteria for accelerated phase (AP) and blast phase (BP) of CML.
Treatment
CML-CP
If patient has very high counts and symptomatic then we admit and give hydration and hydroxyurea or imatinib mesylate to control leukocytosis and thrombocytosis.[8] Dose of hydroxyurea used is 1-6 g/day and dose of imatinib mesylate is 400 mg/day.
CML-AP
Use same strategy as CP except starting dose of imatinib mesylate is 600 mg/day.
CML-BC
If patient present in blast crisis phase from first day then we decide treatment on following criteria:
- Physiological age and performance status (PS),
- Type of blast crisis (myeloid or lymphoid)
- Financial status of patient
- Is candidate for allo-bone marrow transplant?
If patient is old with poor PS then we start only imatinib 600 mg with supportive care and change dose of imatinib according to response.
If patient has good performance score with lymphoid crisis then we start imatinib 600 mg/day with MCP 841 protocol and change dose of imatinib according to response.
Patients with myeloid crisis treated with 7 + 3 induction chemotherapy and imatinib 600 mg/day if no financial constraints; otherwise we give Ara-C subcutaneous with imatinib.
Monitoring of responses to imatinib: Standard Recommendations were slighltly modified as shown in Table 1.
Table 1
Recommendations for monitoring individual patients
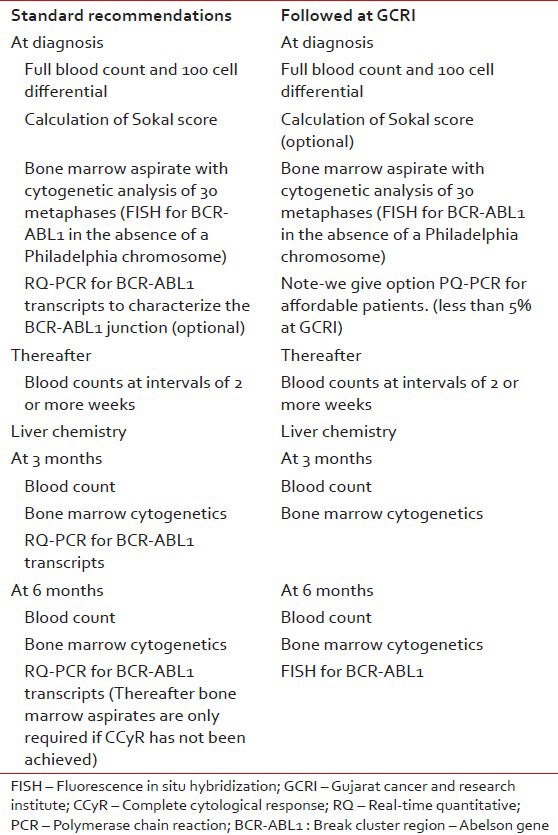
The first evidence of response should be reduction of the excessive leukocyte count, followed then by normalization of a more sensitive measure of residual leukemia, namely the number of Ph-positive metaphases in the bone marrow. The most sensitive available test for low levels of leukemia is to measure BCR-ABL1 transcript numbers in the blood or marrow using a real time quantitative reverse transcriptase PCR (RQ-PCR) (not available at our center). The use of FISH to identify a BCR-ABL1 fusion gene in interphase cells is more sensitive than metaphase cytogenetics, but less sensitive than a RQ-PCR.
WHEN WE CHANGE DOSE OF IMATINIB
We follow recommendations of European Leukemia Net (2006) for defining imatinib failure [Table 2].
Table 2
Criteria for definition of “failure” based on European leukemia net recommendations (2006) and criteria for “optimal response” based on European leukemia net recommendations (2009)
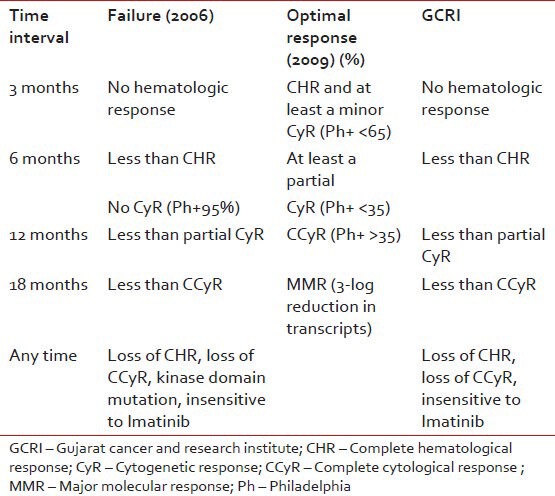
We do conventional cytogenetics at every 6 months interval; if report is inconclusive due to the absence of metaphase cell then we repeat it.
If patient comes under definition of imatinib failure then we increase dose of imatinib from 400 mg to 600 mg and further to 800 mg. Most of patients at our center are not affording for second generation tyrosine kinase inhibitors.
Dasatanib: We have treated only 8 patients with Dasatanib mainly those patients who progressed on imatinib. 2 patients have achieved long-term hematological remission and are presently taking Dasatanib. One patient is in Molecular complete response (CR).
If patient not responding to maximum dose of imatinib or does not tolerate then change to hydroxyurea.
Allo-SCT
In both lymphoid and myeloid blast crisis treated as above, the probability of relapse is high and this risk may be reduced by allo-SCT carried out while the patient is in apparent remission. At our center, if patient with blast transformation has suitable match with financial support preferred treatment after remission is allo-SCT.
All 4 patients have undergone allo-BMT at our center for CML, 2 of them are in CR now. 1 patient expired after 6 months due to chronic graft versus host disease. One patient is undergoing RIC transplantation.
IMATINIB AND PREGNANCY
Imatinib could be teratogenic in certain circumstances, so women taking the drug have been routinely advised to take steps to avoid conception. Nonetheless, some women have conceived while taking imatinib and in most cases where the pregnancy went to term the baby appears to have been normal. Certain specific developmental abnormalities including hypospadias, exomphalos and defective skeletal formation have been seen more often than would have been expected in women not taking imatinib[9] and the advice to avoid pregnancy while being treated with the drug must be upheld.
Little is known about the possible harmful effect of imatinib on male spermatogenesis, so again no firm recommendations can be offered to the potential father treated with i matinib.
RESULTS
Data from GCRI
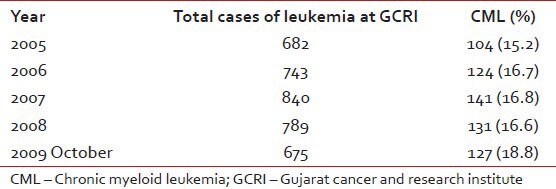
CML accounts for 16.6% of cases of leukemias and about 1% of all cancers at our center (GCRI). The year wise registration of the patients is shown in Table 3. Sex ratio at GCRI-Male-58% and Female-42%. Only 3% cases belong to Pediatric age group (0-15 year) and 2% cases to geriatric group (>65 year). The distribution of the patients as per phase of CML at presentation is shown in Figure 1.
Table 3
Incidence of CML cases at GCRI
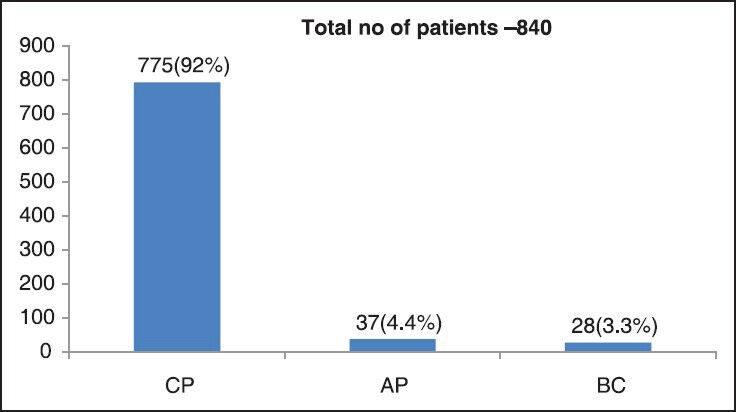
| Fig. 1 Phase of CML at presentation
SIDE EFFECTS
Patients taking imatinib are having less unpleasant side-effects associated with conventional cytotoxic drugs. Among the most prominent non-hematologic side-effects are nausea, fluid retention, weight gain, diarrhea, bone pains, rashes and disturbances of liver function. Imatinib can also cause significant cytopenias.
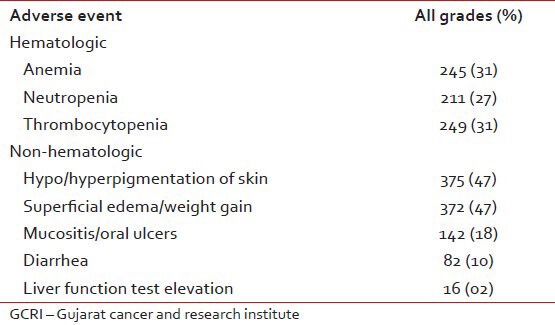
Patients with anemia may benefit from administration of erythropoietin and those with neutropenia may be able to tolerate imatinib at full dosage if supported with granulocyte colony-stimulating factor;[10,11] severe thrombocytopenia may, however, necessitate dose reduction or a change to another agent. At GCRI out of 792 evaluable patients 10 patients developed Grade IV thrombocytopenia (0.1%), 7 patients had Grade IV neutropenia (0.1%), 7 patients had pancytopenia (0.1%), 08 patients had Grade IV rashes and 5 patients developed disturbances of liver function as shown in Table 4.
Table 4
Adverse event of imatinib noted at GCRI
RESPONSE
- Patients achieving CHR – 96%
- Mean time to achieve CHR – 2.03 months
- CHR in less than 3 months – 87%
- Patients achieving CCyR – 36%
- Mean time to achieve CCyR – 17.25 months
- Patients achieving partial CyR (PCyR) – 23%
- Mean time to achieve PCyR – 13.08 months.
CONCLUSION
CML mainly present in CP in our patient population and 96% achieved CHR. The CCyR was seen in approximately 36% of patients which was slightly less than compared to patients in IRIS study where 57% had shown CCyR. Around 30-50% population has some toxicity but Grade IV toxicity requiring intervention was present in less than 1% cases.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Phase of CML at presentation


 PDF
PDF  Views
Views  Share
Share

