The Role of Mutation Testing in Patients with Chronic Myeloid Leukemia in Chronic Phase after Imatinib Failure and Their Outcomes after Treatment Modification: Single institutional Experience Over 13 Years
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 328-333
DOI: DOI: 10.4103/ijmpo.ijmpo_115_17
Abstract
Introduction: BCR-ABL1 kinase domain mutations represent the most frequent mechanism of resistance to tyrosine kinase inhibitor (TKI) therapy, being detected in 40%–50% of imatinib-resistant patients with chronic myeloid leukemia in chronic phase (CML-CP). Over 100 BCR-ABL1 single-point mutations have been reported in patients with imatinib-resistant CML. There were few studies reported from India on BCR-ABL kinase mutations in imatinib failure patients. We present our data on imatinib resistance mutation analysis (IRMA) and use of imatinib dose hike and 2nd-generation TKI at our institute. Materials and Methods: All patients with a diagnosis of CML in a university hospital from June 2003 to July 2016 and who were tested for IRMA in view of imatinib failure, those in CP, and age <18 class="b" xss=removed>Results: A total of 2110 cases of CML reviewed and 269 cases of CML with imatinib failure were analyzed. The male to female ratio was 1.7:1. The median age at presentation was 36 years (range: 18–66 years). Among these, 26% were primary failures and 74% were secondary failures. The treatment was modified either as imatinib dose hike or nilotinib/dasatinib. Molecular response at 12 months was achieved in 25.7% in imatinib dose hike, 46.6% in nilotinib, and 53.8% in dasatinib arms. The 4-year overall survival in mutation detected group was 37.5% and in nonmutated group was 87.7%. Conclusion: Imatinib-resistant mutations were more common in the cases with secondary failure though not statistically significant. T315I mutation was the common mutation found in the study. Imatinib dose hike to the failure cases resulted in optimal hematological response rates.
Keywords
2nd-generation tyrosine kinase inhibitor - chronic myeloid leukemia - imatinib resistance mutation analysisPublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
BCR-ABL1 kinase domain mutations represent the most frequent mechanism of resistance to tyrosine kinase inhibitor (TKI) therapy, being detected in 40%–50% of imatinib-resistant patients with chronic myeloid leukemia in chronic phase (CML-CP). Over 100 BCR-ABL1 single-point mutations have been reported in patients with imatinib-resistant CML. There were few studies reported from India on BCR-ABL kinase mutations in imatinib failure patients. We present our data on imatinib resistance mutation analysis (IRMA) and use of imatinib dose hike and 2nd-generation TKI at our institute.
Materials and Methods:
All patients with a diagnosis of CML in a university hospital from June 2003 to July 2016 and who were tested for IRMA in view of imatinib failure, those in CP, and age <18>
Results:
A total of 2110 cases of CML reviewed and 269 cases of CML with imatinib failure were analyzed. The male to female ratio was 1.7:1. The median age at presentation was 36 years (range: 18–66 years). Among these, 26% were primary failures and 74% were secondary failures. The treatment was modified either as imatinib dose hike or nilotinib/dasatinib. Molecular response at 12 months was achieved in 25.7% in imatinib dose hike, 46.6% in nilotinib, and 53.8% in dasatinib arms. The 4-year overall survival in mutation detected group was 37.5% and in nonmutated group was 87.7%.
Conclusion:
Imatinib-resistant mutations were more common in the cases with secondary failure though not statistically significant. T315I mutation was the common mutation found in the study. Imatinib dose hike to the failure cases resulted in optimal hematological response rates.
Introduction
BCR-ABL protein, which results from a reciprocal translocation involving chromosomes 9 and 22, has a central role in the pathogenesis of chronic myeloid leukemia (CML). The BCR-ABL protein functions as a constitutively activated tyrosine kinase and the drugs are developed to specifically inhibit the BCR-ABL tyrosine kinase.[1]
BCR-ABL1 kinase domain mutations are the most frequent mechanism of resistance to tyrosine kinase inhibitor (TKI) therapy, which is detected in 40%–50% of imatinib-resistant patients with CML in chronic phase (CML-CP[2,3]). More than 100 BCR-ABL1 single-point mutations have been detected in patients with imatinib-resistant CML, which led to various levels of TKI resistance.[4] A threonine to isoleucine substitution at the 315 residue (T315I), which forms a key H-bond interaction with TKIs, results in clinical insensitivity to imatinib, nilotinib, and dasatinib.[2,5,6,7,8,9,10] Other mutations can be inhibited to some extent by 2nd-generation TKIs (SG-TKIs).
The different mutations may therefore require different strategies to overcome resistance such as dose escalation for those that confer moderate resistance or shifting to SG-TKIs or even transplantation for more resistant mutations.[11]
Aims and objectives
The aim of this study was to analyze BCR-ABL tyrosine kinase domain mutations in CML patients with imatinib failure and study the outcomes with imatinib dose escalation or SG-TKIs following imatinib resistance mutation analysis (IRMA).
Materials and Methods
All patients with a diagnosis of CML in tertiary care university hospital in South India between 2003 and July 2016 and a minimum follow-up of 12 months were included in the study. European LeukemiaNet (ELN) recommendations were used for the diagnosis and monitoring the response to treatment.[12] Sokal, Euro, and EUTOS scoring systems were used for risk stratification.[13,14,15] Primary failure was defined as failure to achieve a given response at a given time and secondary failure as loss of initial response to TKI at anytime.
All patients who were tested for IRMA in view of imatinib failure, those in CP, and age <18>
Fisher's exact test using Prism version 7.02, Graphpad software, LaJolla, California, USA was used to analyze the proportion of mutations depending on the type of failure. To evaluate the association between mutation status and survival, log-rank test was used. Survival probabilities were estimated by the Kaplan–Meier method and compared by the log-rank test.[16]
Results
A total of 2110 patients of CML reviewed and 269 cases of CML with imatinib failure were analyzed. Baseline characteristics of these patients (n = 269) were tabulated in Table 1.
Table 1
Baseline characteristics (n=269)

|
The baseline hematological parameters assessed for these patients were represented in Table 2.
Table 2
Baseline hematological parameters

|
Molecular studies for BCR-ABL revealed the major transcript (p210 = e13a2, e14a2) in 138 cases, and in 131 cases, the transcript was not specified. Transcript type found in the cases is shown in Table 3.
Table 3
Molecular studies for BCR-ABL

|
The patients were risk stratified using three prognostic score systems – Sokal, Hasford, and EUTOS. There were some patients for whom risk stratification could not be done because of paucity of data which was depicted in Table 4.
Table 4
Risk stratification
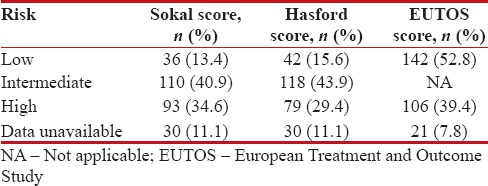
|
The median duration of imatinib treatment before developing treatment resistance was 24 months (range: 3–60 months).
Based on the therapeutic response to imatinib, patients were categorized into primary and secondary failure taking into consideration the ELN guidelines. Both primary and secondary failures are subcategorized into hematological, cytogenetic, and molecular resistance. Categorization of imatinib failure is shown in Table 5. Of the 269 cases tested for imatinib resistance, mutations were detected in 88 (32.7%) cases and 181 (67.3%) cases were negative for mutations. The distribution of mutations in primary and secondary failure was depicted in Table 6. Fisher's exact test did not result in statistical significance with P = 0.0536.
Table 5
Categorization of imatinib failure (n=269)

|
Table 6
Mutation analysis and its distribution in imatinib failure

|
Mutations in kinase domain were detected in 88 (32.7%) cases. Mutations in ATP binding region (P-loop), other P-loop, substrate binding region, SH2 contact, A-loop, C-helix, and others were found in 28 (31.8%), 25 (28.4%), 14 (15.9%), 6 (6.8%), 3 (3.4%), 1 (1.1%), and 11 (12.5%) cases, respectively. In the ATP binding region, T315I mutation was seen in 28 cases (31.8%). Mutations in kinase domain based on the site and amino acid distribution were shown in Figures Figures11 and and22.
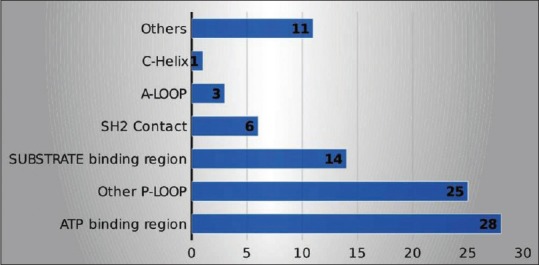
| Figure 1:Mutations in kinase domain based on the site

| Figure 2:Mutations in kinase domain based on amino acid substitution
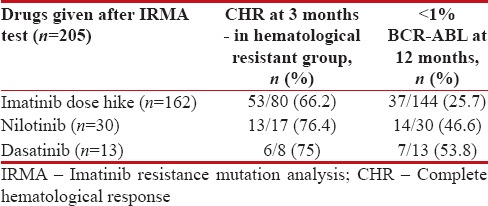
Of the 269 cases evaluated for imatinib resistance, the treatment was modified either as imatinib dose escalation or second-line TKI for 205 cases. The remaining 64 cases were given supportive care. Imatinib dose hike (600/800 mg), nilotinib, and dasatinib were given to 162, 30, and 13 cases, respectively. Response evaluation was done for these cases at 3 months and 12 months. Of 205 cases, 105 were in hematological failure at the time of treatment modification and the remaining 100 cases were in complete hematological response (CHR). These 105 cases were assessed for CHR at 3 months and were achieved in 53 of the 80 cases (66.2%) in imatinib dose hike, 13 of the 17 cases (76.4%) in nilotinib, and 6 of the 8 cases (75%) in dasatinib arms.
Molecular response was assessed in 187 of 205 cases. For 18 cases, molecular response could not be assessed at the end of 1 year of treatment modification. Molecular response (<1 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686977/table/T7/" target="table" class="fig-table-link figpopup" rid-figpopup="T7" rid-ob="ob-T7" co-legend-rid="" xss=removed>Table 7.
Table 7
Response to treatment modification
|
Median OS (since imatinib resistance) for T315I mutation group was 20.5 months (range: 1.4–42.8 months), for P-loop mutation group was 31 months (range: 1.4–57 months), and it was not reached for the group with non-P-loop mutation (3.9 months-NR) and nonmutated groups (5.3 months-NR) with median follow-up of 48 months from the date of imatinib resistance (range: 1.4–96 months).
Four-year OS in mutation detected group was 37.5% and in nonmutated group was 87.7%. OS from the date of imatinib resistance was shown in Figures Figures33 and and44.
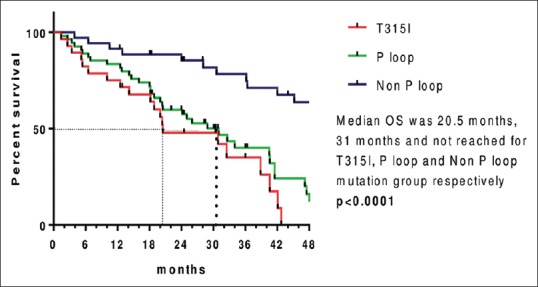
| Figure 3:Kaplan–Meier estimates of overall survival since imatinib resistance between various mutant groups
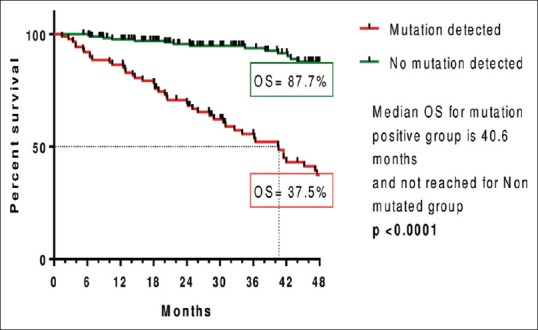
| Figure 4:Kaplan–Meier estimates of overall survival since imatinib resistance between mutated and nonmutated group
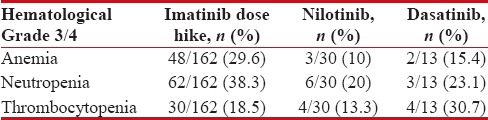
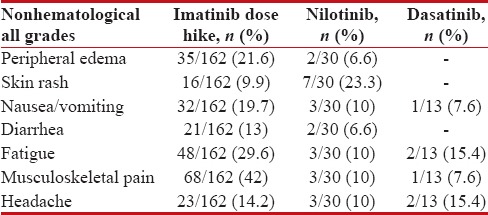
The hematological and nonhematological adverse effects were shown in Tables Tables88 and and99.
Table 8
Hematological adverse effects
|
Table 9
Nonhematological adverse effects
|
Discussion
Our study comprised 269 CML patients with imatinib treatment failure. The median age was 36 years (18-66 years) and the sex ratio was 1.7:1, whereas in other studies by Quintas Cardama Alfonso et al.,[17] Jabbour et al.,[18] Cortes et al.,[19] Rajappa et al.,[20] and Mishra et al.,[21] the median age was 52 years (18–79), 57 years (25–82), 51 years (17–96), 36 years (18–65), and 35 years, respectively. The sex ratio was 1.08:1, 1.6:1, 2.7:1, and 1.6:1 in the studies done by Quintas Cardama Alfonso et al.,[17] Jabbour et al.,[18] Rajappa et al.,[20] and Mishra et al.,[21] respectively.
We stratified patients into different risk groups using three scoring systems; nevertheless, Sokal score was used in most of the studies[18,21,22,23] and in comparison to Western studies, this study had more number of intermediate and high-risk Sokal score.
In this study, the ratio of primary and secondary failure was 1:2.8 and the ratio of cytogenetic/molecular and hematologic failure was 1.57:1. The results of our study were similar to Jabbour et al.[18] which included all three phases of disease. Compared to our study, Jabbour et al.[23] showed higher cytogenetic failures. Rajappa et al.,[20] Cortes et al.,[19] and Kantarjian et al.[25] showed more hematological failures compared to our study.
However, Soverini et al.[24] showed similar ratio of hematological and cytogenetic failure when compared to our study, yet there is difference in the ratio of primary and secondary failure.
Although the frequency of mutations was higher in secondary failure cases, it was not statistically significant (P = 0.38) in this study. Al-Ali et al.[26] and Soverini et al.[24] demonstrated significantly higher frequency of mutations in secondary failure cases. In the study by Jabbour et al.,[18] it was observed that mutations associated with secondary imatinib resistance occur more frequently in later stages of the disease and are associated with older age, prior interferon therapy, initiation of imatinib therapy in AP or BC, development of clonal evolution, high-risk Sokal score at diagnosis, and failure to achieve complete cytogenetic response (CCyR) by 12 months.
T315I was the most commonly seen mutation in our study (31.8%), Rajappa et al.[20] (31%) which was our previous institutional study and Suresh-Babu et al.[27] (16.6%) studies. IRMA was directed to T315I presence or absence for many years at our institute that could have been the reason for high proportion of this mutation in our study.
E255 V/K mutation was the most common in Soverini et al.[24] study (16%) and it was seen in 5.7% of cases in our study. Quintas Cardama Alfonso et al.,[17] Jabbour et al.,[18] and Nicolini et al.[22] showed the most frequent mutation at G250 residue and this was the 3rd most common in our study and Rajappa et al.[20] and Srivastava and Dutt[31] studies.
Treatment modification after imatinib resistance mutation analysis result
In our study, CHR at 3 months to imatinib dose escalation was 66.2%. In various studies on imatinib dose escalation, the CHR at 3 months was between 45% and 86%.[23,25] In our study, CCyR/< 1 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5686977/#ref20" rid="ref20" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_649899322" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>20,23,25]
For 2nd-line TKI, CHR at 3 months was attained in 75% of cases in our study. In other studies,[17,19,28,29,30] CHR at 3 months ranged from 82% to 92%. In this study, CCyR/< 1 xss=removed>nd-line TKI was 46.6% to nilotinib and 53.8% to dasatinib. In other studies, it ranged from 28.4% to 69%.
Survival analysis
In this study, the median OS and range for T315I mutation and P-loop mutation were 20.5 months (range: 1.4–42.8) and 31 months (range: 1.4–57), respectively, and it was not reached for the group with non-P-loop mutation (3.9 months-NR) and nonmutated groups (5.3 months-NR). The 4-year OS in mutation-detected group was 37.5% and in nonmutated group was 87.7%.
Our study showed inferior survival for T315I and P-loop mutation patients compared to those with non-P-loop mutations and with no mutations. Similar results were seen in the study done by Nicolini et al.[22] There was no difference in survival between patients with T315I, other mutations, or no mutations in the study done by Jabbour et al.[30]
In this study, the 4-year OS was inferior in the group with mutations compared to the group with no mutations. Results were similar in the studies done by Quintas Cardama Alfonso et al.[17] and Nicolini et al.[22]
Limitations of the study
The drawbacks of this study were that it is retrospective study. Laboratory data were not available for all patients to stratify the risk. There was underreporting of both hematological and nonhematological adverse events because of inadequate documentation in case records. Second-generation TKI and hematopoietic stem-cell transplantation could not be provided for the eligible cases due to financial constraints.
Imatinib-resistant mutations were more common in the cases with secondary failure though it was not statistically significant. T315I mutation which is resistant to all second-generation TKIs was the common mutation found in the study.
Imatinib dose hike to the failure cases resulted in optimal hematological response rates which is on par with second-generation TKIs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood 2008;112:4808-17.
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001;293:876-80.
- von Bubnoff N, Schneller F, Peschel C, Duyster J. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: A prospective study. Lancet 2002;359:487-91.
- Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 2009;113:1619-30.
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 2001;344:1038-42.
- Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005;7:129-41.
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006;354:2542-51.
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006;354:2531-41.
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino) thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004;47:6658-61.
- ;Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004;305:399-401.
- Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: Does the BCR-ABL mutation status really matter? Blood 2009;114:5426-35.
- Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009;27:6041-51.
- Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 1984;63:789-99.
- Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998;90:850-8.
- Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: The EUTOS score. Blood 2011;118:686-92.
- Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1965;53:457-81.
- strong> Quintás-Cardama A, Kantarjian H, O'Brien S, Jabbour E, Borthakur G, Ravandi F, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica 2011;96:918-21.
- Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O'Brien S, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia 2006;20:1767-73.
- Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O'Brien S, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood 2007;110:4005-11.
- Rajappa S, Mallavarapu KM, Gundeti S, Roshnipaul T, Jacob RT, Digumarti R. Kinase domain mutations and responses to dose escalation in chronic myeloid leukemia resistant to standard dose imatinib mesylate. Leuk Lymphoma 2010;51:79-84.
- Mishra P, Seth T, Mahapatra M, Saxena R. Report of chronic myeloid leukemia in chronic phase from All India Institute of Medical Sciences, 1990-2010. Indian J Med Paediatr Oncol 2013;34:159-63.
- Nicolini FE, Corm S, Lê QH, Sorel N, Hayette S, Bories D, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: A retrospective analysis from the French intergroup of CML (Fi(phi)-LMC GROUP). Leukemia 2006;20:1061-6.
- Jabbour E, Kantarjian HM, Jones D, Shan J, O'Brien S, Reddy N, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood 2009;113:2154-60.
- Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: By the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res 2006;12:7374-9.
- Kantarjian HM, Talpaz M, O'Brien S, Giles F, Garcia-Manero G, Faderl S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 2003;101:473-5.
- Al-Ali HK, Heinrich MC, Lange T, Krahl R, Mueller M, Müller C, et al. High incidence of BCR-ABL kinase domain mutations and absence of mutations of the PDGFR and KIT activation loops in CML patients with secondary resistance to imatinib. Hematol J 2004;5:55-60.
- Suresh-Babu M, Sirsath N, Lakshmaiah K, Babu G, Tagarapura S, Dasappa L. Imatinib resistance mutation analysis: Experience from a tertiary oncology center. Int J Cancer Ther Oncol 2015;3:3022.
- Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood 2011;117:1141-5.
- Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 2010;95:232-40.
- Jabbour E, Kantarjian H, Jones D, Breeden M, Garcia-Manero G, O'Brien S, et al. Characteristics and outcomes of patients with chronic myeloid leukemia and T315I mutation following failure of imatinib mesylate therapy. Blood 2008;112:53-5.
- Srivastava S, Dutt S. Imatinib mesylate resistance and mutations: An Indian experience. Indian J Med Paediatr Oncol 2013;34:213-20.

| Figure 1:Mutations in kinase domain based on the site

| Figure 2:Mutations in kinase domain based on amino acid substitution

| Figure 3:Kaplan–Meier estimates of overall survival since imatinib resistance between various mutant groups

| Figure 4:Kaplan–Meier estimates of overall survival since imatinib resistance between mutated and nonmutated group
References
- Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood 2008;112:4808-17.
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001;293:876-80.
- von Bubnoff N, Schneller F, Peschel C, Duyster J. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: A prospective study. Lancet 2002;359:487-91.
- Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 2009;113:1619-30.
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 2001;344:1038-42.
- Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005;7:129-41.
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006;354:2542-51.
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006;354:2531-41.
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino) thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004;47:6658-61.
- ;Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004;305:399-401.
- Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: Does the BCR-ABL mutation status really matter? Blood 2009;114:5426-35.
- Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009;27:6041-51.
- Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 1984;63:789-99.
- Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998;90:850-8.
- Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: The EUTOS score. Blood 2011;118:686-92.
- Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1965;53:457-81.
- strong> Quintás-Cardama A, Kantarjian H, O'Brien S, Jabbour E, Borthakur G, Ravandi F, et al. Outcome of patients with chronic myeloid leukemia with multiple ABL1 kinase domain mutations receiving tyrosine kinase inhibitor therapy. Haematologica 2011;96:918-21.
- Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O'Brien S, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia 2006;20:1767-73.
- Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O'Brien S, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood 2007;110:4005-11.
- Rajappa S, Mallavarapu KM, Gundeti S, Roshnipaul T, Jacob RT, Digumarti R. Kinase domain mutations and responses to dose escalation in chronic myeloid leukemia resistant to standard dose imatinib mesylate. Leuk Lymphoma 2010;51:79-84.
- Mishra P, Seth T, Mahapatra M, Saxena R. Report of chronic myeloid leukemia in chronic phase from All India Institute of Medical Sciences, 1990-2010. Indian J Med Paediatr Oncol 2013;34:159-63.
- Nicolini FE, Corm S, Lê QH, Sorel N, Hayette S, Bories D, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: A retrospective analysis from the French intergroup of CML (Fi(phi)-LMC GROUP). Leukemia 2006;20:1061-6.
- Jabbour E, Kantarjian HM, Jones D, Shan J, O'Brien S, Reddy N, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood 2009;113:2154-60.
- Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: By the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res 2006;12:7374-9.
- Kantarjian HM, Talpaz M, O'Brien S, Giles F, Garcia-Manero G, Faderl S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood 2003;101:473-5.
- Al-Ali HK, Heinrich MC, Lange T, Krahl R, Mueller M, Müller C, et al. High incidence of BCR-ABL kinase domain mutations and absence of mutations of the PDGFR and KIT activation loops in CML patients with secondary resistance to imatinib. Hematol J 2004;5:55-60.
- Suresh-Babu M, Sirsath N, Lakshmaiah K, Babu G, Tagarapura S, Dasappa L. Imatinib resistance mutation analysis: Experience from a tertiary oncology center. Int J Cancer Ther Oncol 2015;3:3022.
- Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz J, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood 2011;117:1141-5.
- Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 2010;95:232-40.
- Jabbour E, Kantarjian H, Jones D, Breeden M, Garcia-Manero G, O'Brien S, et al. Characteristics and outcomes of patients with chronic myeloid leukemia and T315I mutation following failure of imatinib mesylate therapy. Blood 2008;112:53-5.
- Srivastava S, Dutt S. Imatinib mesylate resistance and mutations: An Indian experience. Indian J Med Paediatr Oncol 2013;34:213-20.


 PDF
PDF  Views
Views  Share
Share

