The Relationship between Serum Selenium and Zinc with Gastroesophageal Cancers in the Southeast of Iran
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 169-172
DOI: DOI: 10.4103/ijmpo.ijmpo_83_16
Abstract
Background: Selenium (Se) and zinc (Zn) have antioxidant and anticancer properties. Objective: The aim of this study was to evaluate the serum levels of Se and Zn and the correlation between the levels of these two elements with risk of incidence of esophageal cancer (EC) and gastric cancer (GC). Materials and Methods: In a case–control study, we selected sixty patients with GC or EC as the intervention group and 120 age-matched individuals as the control group. Exclusion criteria were the individuals with kidney and liver failure and the consumer of dietary supplements such as Se and Zn. Measurement of serum Se was done in a graphite furnace system and atomic absorption device of Varian and of serum Zn was done by a flame photometer system (flame) and atomic absorption device of Varian. Results: In thirty patients of ECs, 90% were squamous cell carcinoma and 10%- adenocarcinoma, and out of thirty patients of GCs, 90%-were intestinal type and 10%-diffuse type. The level of two elements in cancer patients was lower than the control group (P < 0> Conclusion: Our study confirmed the findings from previous prospective studies and randomized trials that reducing of lower levels of Se and Zn can effect on incidence of cancer.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Selenium (Se) and zinc (Zn) have antioxidant and anticancer properties.
Objective:
The aim of this study was to evaluate the serum levels of Se and Zn and the correlation between the levels of these two elements with risk of incidence of esophageal cancer (EC) and gastric cancer (GC).
Materials and Methods:
In a case–control study, we selected sixty patients with GC or EC as the intervention group and 120 age-matched individuals as the control group. Exclusion criteria were the individuals with kidney and liver failure and the consumer of dietary supplements such as Se and Zn. Measurement of serum Se was done in a graphite furnace system and atomic absorption device of Varian and of serum Zn was done by a flame photometer system (flame) and atomic absorption device of Varian.
Results:
In thirty patients of ECs, 90%-were squamous cell carcinoma and 10adenocarcinoma, and out of thirty patients of GCs, 90%-were intestinal type and 10%-diffuse type. The level of two elements in cancer patients was lower than the control group (P < 0>
Conclusion:
Our study confirmed the findings from previous prospective studies and randomized trials that reducing of lower levels of Se and Zn can effect on incidence of cancer.
Introduction
Esophageal cancer (EC) is the eighth most common cancer with respect to incidence and the sixth most common cancer with respect to mortality worldwide.[1] This cancer is classified into two main types histologically: esophageal adenocarcinoma (EA) and esophageal squamous cell carcinoma (ESCC), each having different risk factors.[2] Despite recent decline, gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer-related death worldwide[3] that is the most common gastrointestinal (GI) cancer in Iran.[4] In blood, selenium (Se) is found in plasma as well as in the cellular compartment where concentrations are higher and the concentrations are highest in tissues such as liver, kidneys, and spleen.[5] Se is from the group of VI elements, which has been studied for antioxidant and anticancer properties,[6,7] especially against GC.[8] Zinc (Zn) is trace element found in blood, and most of it is contained in bones, skin, and hair (~70%), with the remainder mainly in liver, kidneys, and muscle. In plasma, one-third of Zn is tightly bound to α2-macroglobulin, the remainder more loosely to albumin.[5] Zn deficiency adversely affects the immune system, increases oxidative stress, increases the generation of inflammatory cytokines,[9] and also potentiates the effects of certain nitrosamines that act as esophageal carcinogens.[10]
The aim of this study was to evaluate the serum levels of Se and Zn and the correlation between the levels of these two elements with risk of incidence of EC and GC in Southeastern Iran.
Materials and Methods
Patients
This study was approved by the Ethics Committee of Zahedan University of Medical Sciences, Zahedan, Iran, at Winter 2016 (Code: 6000988). In a case–control study, we selected sixty patients with EC or GC as the intervention group who referred to Clinic of Oncology, Emam Ali Hospital, as the intervention group and also 120 age-matched individuals as the control group referred patients to the Orthopedic Ward of Khatam-al-Anbia Hospital who had just trauma or fracture.
Exclusion criteria: The individuals with kidney and liver failure and the consumer of dietary supplements such as Se and Zn.
Methods of measurement
Measurement of serum Se was done by a graphite furnace system and atomic absorption device of Varian (Varian 240FS, Co., USA) in Laboratory of Toxicology, Razavi Hospital, Mashhad, Iran. System wavelength was 196 nm and width of the valve was 0.5 nm. The standard had been made by the Merc Company with concentration between 50 and 500 μg/l. We used a calibration standard edition that palladium chloride was used as a matrix modifier.
Measurement of serum Zn was done by a flame photometer system (flame) and atomic absorption device of Varian (wavelength of 213 nm). The standard for Zn had been made by the Merck Company.
Statistical analysis
Data analysis was done by the SPSS version 16 (SPSS, Inc., Chicago, IL, USA) (Student's t-test for comparison between the means and Chi-square test for sex).
Results
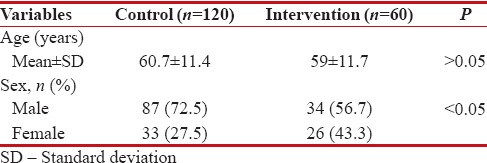
The mean age and the prevalence of sex for two groups are presented in Table 1. There was no significant difference for age in two groups (P > 0.05), but there was a significant difference for sex (P < 0>
Table 1
The baseline characteristics of the intervention and control groups
|
Table 2 shows the type of pathology for the intervention group. Out of sixty patients, thirty patients (50%) had EC (ESCC and EA in 90%-and 10%, respectively) and thirty patients (50%) had GC (intestinal type and diffuse type in 90%-and 10%, respectively).
Table 2
Type of pathology for intervention group

|
The serum level of the variables in the intervention and control groups is presented in Table 3. There was significant difference between two groups for level of Se and also for the level of Zn (P < 0 xss=removed>.001). Therefore, the level of two elements in cancer patients is lower than the control group.
Table 3
The serum level of the variables in intervention and control groups

|
The serum levels of Se and Zn is located in Table 4 based on age groups. There was a significant difference between the level of Zn in two groups in all age groups and for level of Se was just in age group of 45–65 years.
Table 4
The serum level of the variables in intervention and control groups based on age ranges

|
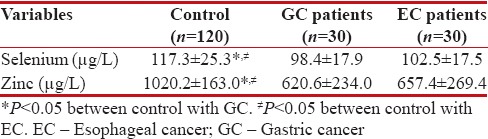
The serum level of the variables in cancer groups and control group is given in Table 5. There was no significant different between two cancer groups for level of Se and Zn (P > 0.05), but there was a significant difference between control groups with two other groups (P < 0>
Table 5
The serum level of the variables in cancer groups and control groups
|
The serum level of the variables in cancer groups and control group based on sex is presented in Figure 1. The levels of Se and Zn in males are lower than females in EC patients, whereas in two other groups are vice versa; there was no significant difference between serum Se and Zn concentrations and sex in the patient groups (P > 0.05).
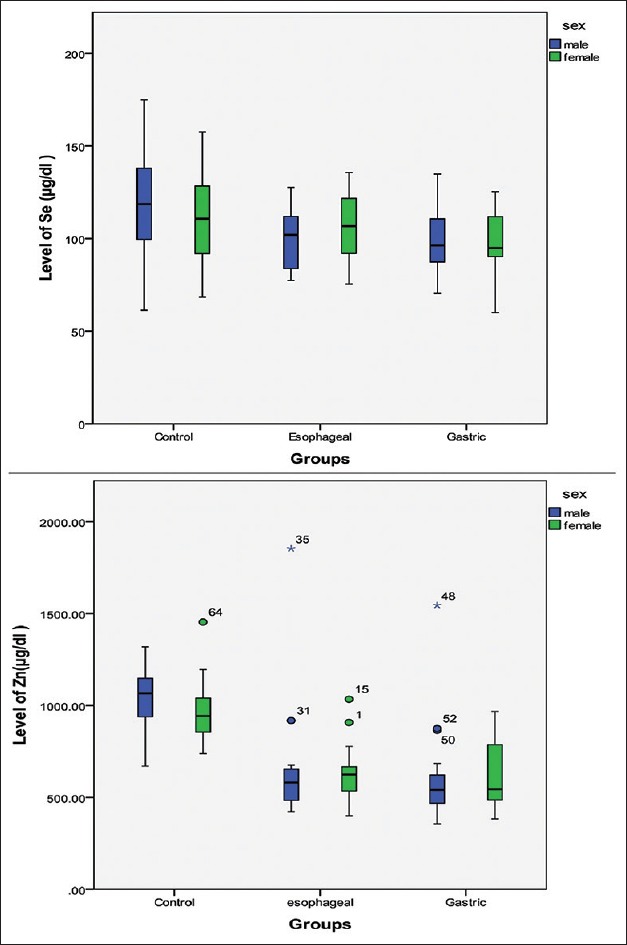
| Figure 6:The serum level of the variables in cancer groups and control groups based on sex
Discussion
GC is the second most common GI malignancy with a male/female ratio of 2.1/1,[11] and EC is the most common GI malignancies in Zahedan (Southeastern Iran)[12] that this pattern is different from other regions in Iran that GC is the most common GI malignancies.[11,12] The highest incidences for EC were reported in China, the Caspian region of Iran, South Africa, and France.[13] Therefore, in this study, we checked the levels of Se and Zn in GC and EC patients in Southeastern Iran compared with normal or control group as reported risk factors for incidence of GI malignancies.
The studies have shown that Se may have anti-carcinogenic effects, especially against cancers of the lung, prostate, skin, and GI system.[14,15] Another study[16] indicated that the inverse association between Se level and GC may occur only among populations with low Se levels. One study[17] observed significant low serum levels of Zn and Se, as compared with normal healthy controls. This shows an association of serum Se and Zn with cancer esophagus. Ji et al.[18] reported that the serum Zn level was lower in advanced GC and also the serum Se level was highly correlated with the location of GC. Rhee et al.[19] reported that the mean Se concentration in cancer patient group (GC and colon cancer cases) was significantly lower than the normal controls. In Iran, the average serum Se in Ardabil, Kerman, Mazandaran, and Golestan was 82, 119, 123, and 155 μg/L, respectively. Therefore, it was suggested that the high incidence of GC and preneoplastic gastric lesions in Ardabil Province could be partly due to low level of Se.[8] Mark et al.[20] found highly significant inverse associations of serum Se levels with the incidence of esophageal and gastric cardia cancers. Abnet et al.[10] reported that concentration of high tissue Zn concentration was strongly associated with a reduced risk of developing ESCC. One study[21] showed that there was an inverse relationship between Se intake and cancer mortality in humans, but there was no correlation between serum/tissue Se concentration and disease histological type or sex in the patient group. Zhang et al.[22] reported that whatever serum levels of Zn were significantly reduced in gastritis, peptic ulcer, and GC group, compared with healthy controls, but the higher the Zn levels are, the more increased risk of GC. The researchers in a review study[11] concluded that an inverse association between Zn concentrations and EC incidence was apparent in most of the reviewed observational studies. In our study, the level of Se and Zn was significantly lower in cancer patients compared with control group or normal group that this study confirmed the results of other studies. In addition, there was no significant difference between serum Se and Zn concentrations and sex in the patient groups, but there was a significant difference between the level of Zn in two groups in three age groups and for level of Se was just in age group of 45–65 years. Furthermore, the levels of two elements were similar in GC compared with EC (P > 0.05).
Conclusion
Our study confirmed findings from previous prospective studies and randomized trials that reducing of lower levels of Se and Zn can effect on incidence of cancer. Therefore, consideration should be given to supplements that contain Se and Zn for the general population and also patients with esophageal and GCs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.
- Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27-57, vii.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62.
- Hosseini Nezhad Z, Darvish Moghaddam S, Zahedi MJ, Hayatbakhsh M, Sharififar F, Ebrahimi Meimand F, et al. Serum selenium level in patients with gastric non-cardia cancer and functional dyspepsia. Iran J Med Sci 2015;40:214-8.
- Biesalski HK, Grimm P. Pocket Atlas of Nutrition. 3rd ed. Stuttgart: Georg Thieme Verlag; 2005.
- Ramoutar RR, Brumaghim JL. Antioxidant and anticancer properties and mechanisms of inorganic selenium, oxo-sulfur, and oxo-selenium compounds. Cell Biochem Biophys 2010;58:1-23.
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med 2003;349:366-81.
- Nouarie M, Pourshams A, Kamangar F, Sotoudeh M, Derakhshan MH, Akbari MR, et al. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J Gastroenterol 2004;10:2544-6.
- Prasad AS, Beck FW, Snell DC, Kucuk O. Zinc in cancer prevention. Nutr Cancer 2009;61:879-87.
- Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301-6.
- Mashhadi MA, Nezam K, Abdollahinejad MJ. Gastric cancer in South East of Iran. Int J Hematol Oncol Stem Cell Res 2009;3:38-42.
- Mashhadi MA, Nezam K, Bakhshipour AR, Ttorbati TF, Ansarimoghaddam AR. High prevalence of esophageal cancer in South East of Iran. Int J Hematol Oncol Stem Cell Res 2011;5:18-21.
- Islami F, Kamangar F, Aghcheli K, Fahimi S, Semnani S, Taghavi N, et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer 2004;90:1402-6.
- Rayman MP. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc Nutr Soc 2005;64:527-42.
- Lee SR, Bar-Noy S, Kwon J, Levine RL, Stadtman TC, Rhee SG. Mammalian thioredoxin reductase: Oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci U S A 2000;97:2521-6.
- Koriyama C, Campos FI, Yamamoto M, Serra M, Carrasquilla G, Carrascal E, et al. Toenail selenium levels and gastric cancer risk in Cali, Colombia. J Toxicol Sci 2008;33:227-35.
- Goyal MM, Kalwar AK, Vyas RK, Bhati A. A study of serum zinc, selenium and copper levels in carcinoma of esophagus patients. Indian J Clin Biochem 2006;21:208-10.
- Ji JH, Shin DG, Kwon Y, Cho DH, Lee KB, Park SS, et al. Clinical correlation between gastric cancer type and serum selenium and zinc levels. J Gastric Cancer 2012;12:217-22.
- Rhee JK, Chung JH, Sakong J, Kang PS, Kim CY, Lee KS, et al. Association between cancer and selenium concentration in blood and toenails. Yeungnam Univ J Med 1992;9:29-43.
- Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst 2000;92:1753-63.
- Charalabopoulos K, Kotsalos A, Batistatou A, Charalabopoulos A, Peschos D, Vezyraki P, et al. Serum and tissue selenium levels in gastric cancer patients and correlation with CEA. Anticancer Res 2009;29:3465-7.
- Zhang WH, Wu XJ, Niu JX, Yan H, Wang XZ, Yin XD, et al. Serum zinc status and Helicobacter pylori infection in gastric disease patients. Asian Pac J Cancer Prev 2012;13:5043-6.

| Figure 6:The serum level of the variables in cancer groups and control groups based on sex
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.
- Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27-57, vii.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62.
- Hosseini Nezhad Z, Darvish Moghaddam S, Zahedi MJ, Hayatbakhsh M, Sharififar F, Ebrahimi Meimand F, et al. Serum selenium level in patients with gastric non-cardia cancer and functional dyspepsia. Iran J Med Sci 2015;40:214-8.
- Biesalski HK, Grimm P. Pocket Atlas of Nutrition. 3rd ed. Stuttgart: Georg Thieme Verlag; 2005.
- Ramoutar RR, Brumaghim JL. Antioxidant and anticancer properties and mechanisms of inorganic selenium, oxo-sulfur, and oxo-selenium compounds. Cell Biochem Biophys 2010;58:1-23.
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med 2003;349:366-81.
- Nouarie M, Pourshams A, Kamangar F, Sotoudeh M, Derakhshan MH, Akbari MR, et al. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J Gastroenterol 2004;10:2544-6.
- Prasad AS, Beck FW, Snell DC, Kucuk O. Zinc in cancer prevention. Nutr Cancer 2009;61:879-87.
- Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97:301-6.
- Mashhadi MA, Nezam K, Abdollahinejad MJ. Gastric cancer in South East of Iran. Int J Hematol Oncol Stem Cell Res 2009;3:38-42.
- Mashhadi MA, Nezam K, Bakhshipour AR, Ttorbati TF, Ansarimoghaddam AR. High prevalence of esophageal cancer in South East of Iran. Int J Hematol Oncol Stem Cell Res 2011;5:18-21.
- Islami F, Kamangar F, Aghcheli K, Fahimi S, Semnani S, Taghavi N, et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer 2004;90:1402-6.
- Rayman MP. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc Nutr Soc 2005;64:527-42.
- Lee SR, Bar-Noy S, Kwon J, Levine RL, Stadtman TC, Rhee SG. Mammalian thioredoxin reductase: Oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci U S A 2000;97:2521-6.
- Koriyama C, Campos FI, Yamamoto M, Serra M, Carrasquilla G, Carrascal E, et al. Toenail selenium levels and gastric cancer risk in Cali, Colombia. J Toxicol Sci 2008;33:227-35.
- Goyal MM, Kalwar AK, Vyas RK, Bhati A. A study of serum zinc, selenium and copper levels in carcinoma of esophagus patients. Indian J Clin Biochem 2006;21:208-10.
- Ji JH, Shin DG, Kwon Y, Cho DH, Lee KB, Park SS, et al. Clinical correlation between gastric cancer type and serum selenium and zinc levels. J Gastric Cancer 2012;12:217-22.
- Rhee JK, Chung JH, Sakong J, Kang PS, Kim CY, Lee KS, et al. Association between cancer and selenium concentration in blood and toenails. Yeungnam Univ J Med 1992;9:29-43.
- Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst 2000;92:1753-63.
- Charalabopoulos K, Kotsalos A, Batistatou A, Charalabopoulos A, Peschos D, Vezyraki P, et al. Serum and tissue selenium levels in gastric cancer patients and correlation with CEA. Anticancer Res 2009;29:3465-7.
- Zhang WH, Wu XJ, Niu JX, Yan H, Wang XZ, Yin XD, et al. Serum zinc status and Helicobacter pylori infection in gastric disease patients. Asian Pac J Cancer Prev 2012;13:5043-6.


 PDF
PDF  Views
Views  Share
Share

