The correlation between Ki-67 with other prognostic factors in breast cancer: A study in Iranian patients
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(02): 95-99
DOI: DOI: 10.4103/0971-5851.180136
Abstract
Context: Despite the fact that breast cancer (BC) is a major health issue, very few studies describe its characteristics in the Middle East. Aim: The aim of this study was to evaluate the use and value of Ki-67 as a prognostic marker in BC and associations between Ki-67, clinical, and histopathological parameters were evaluated. Subjects and Methods: In a retrospective study, 260 BC women and invasive ductal carcinoma were included to our study in Kermanshah city, Iran. Age, tumor size, lymph node involvement, histological grade, nuclear grade, and vascular invasion were other factors that determined in a lot of patients. Results: The mean age at diagnosis was 47.6 years (range, 24-84 years) with 100% female. Of 243 patients that tumor size was determined for them, 207 patients (85.2%) had tumor size ≥ 2 cm, and 36 patients (14.8%) had size < 2 cm and also of 237 patients, 47 patients (19.8%), 140 (59.1%), and 50 (21.1%) had histological grades I, II, and III, respectively. There is significant correlation between Ki-67 with nuclear grade, human epidermal growth factor receptor 2 (HER2), and p53 (P < 0.05). Based on this result, more patients with Ki-67 ≥ 20% have higher nuclear grade, p53-positive, and HER2-positive. There was correlation between Ki-67 with type of tumor (P = 0.009). Conclusions: The higher Ki-67 has a direct significant correlation with higher nuclear grade, p53-positive, and HER2-positive. Furthermore, triple negative patients have higher Ki-67 compared to other subtypes.
Keywords
Breast cancer - histological grade - Ki-67 - P53Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
Despite the fact that breast cancer (BC) is a major health issue, very few studies describe its characteristics in the Middle East.
Aim:
The aim of this study was to evaluate the use and value of Ki-67 as a prognostic marker in BC and associations between Ki-67, clinical, and histopathological parameters were evaluated.
Subjects and Methods:
In a retrospective study, 260 BC women and invasive ductal carcinoma were included to our study in Kermanshah city, Iran. Age, tumor size, lymph node involvement, histological grade, nuclear grade, and vascular invasion were other factors that determined in a lot of patients.
Results:
The mean age at diagnosis was 47.6 years (range, 24-84 years) with 100% female. Of 243 patients that tumor size was determined for them, 207 patients (85.2%) had tumor size ≥2 cm, and 36 patients (14.8%) had size < 2 cm and also of 237 patients, 47 patients (19.8%), 140 (59.1%), and 50 (21.1%) had histological grades I, II, and III, respectively. There is significant correlation between Ki-67 with nuclear grade, human epidermal growth factor receptor 2 (HER2), and p53 (P < 0.05). Based on this result, more patients with Ki-67 ≥20% have higher nuclear grade, p53-positive, and HER2-positive. There was correlation between Ki-67 with type of tumor (P = 0.009).
Conclusions:
The higher Ki-67 has a direct significant correlation with higher nuclear grade, p53-positive, and HER2-positive. Furthermore, triple negative patients have higher Ki-67 compared to other subtypes.
INTRODUCTION
Breast cancers (BCs) are the most frequent malignancy among women that can be a leading cause of death through middle-aged women. Despite the fact that BC is a major health issue, very few studies describe its characteristics in the Middle East.[1] BCs can be divided based on their gene expression profiles, into at least four groups: Luminal-type, the human epidermal growth factor receptor 2 (HER2)-type, normal-like-type, and basal-type. Luminal-type cancers are characterized by an activated estrogen receptor (ER) signaling pathway and are divided into two subtypes, luminal subtypes “A” and “B”. In general, luminal-subtype-A tumors express higher levels of ER and carry a better prognosis than do luminal-subtype-B tumors.[2] ER positivity predicts response to endocrine therapy such as antiestrogen (tamoxifen) and trastuzumab therapy (herceptin) for tumor with HER2 overexpression.[3] Ki-67 is a nuclear antigen, which exists in proliferative cells. A number of studies have shown that the immune response of Ki-67 is closely associated with the cell cycle. Furthermore, Ki-67 may predict the pathological remission rate in BC patients following neoadjuvant chemotherapy, as an increased Ki-67 level following neoadjuvant chemotherapy indicates a poor prognosis.[4] A meta-analysis involving 12, 155 patients demonstrated that the Ki-67 positivity confers a higher risk of recurrence and a worse survival rate in patients with early BC. Even though this meta-analysis could not scrutinize if Ki-67 had independent prognostic value beyond the standard clinicopathological variables, it confirmed that the high levels of Ki-67 are associated with worse prognoses.[5] The other important biological markers in early BC are tumor size, nuclear grade, axillary lymph node involvement, ER, progesterone receptor (PR), and HER2 status. Uncontrolled proliferation (such as Ki-67) is a key characteristic of malignant tumors and, therefore, tumor proliferation is one of the major factors associated with prognosis.[6,7]
The aim of this study was to evaluate the use and value of Ki-67 as a prognostic marker in BC and associations between Ki-67, clinical, and histopathological parameters were evaluated.
SUBJECTS AND METHODS
Patients
In a retrospective study, 260 BC women and invasive ductal carcinoma were included to our study in Kermanshah City, Iran. They received chemotherapy, radiotherapy, or hormone therapy. A lot of patients underwent primary surgery. A sufficient sample size was selected from any patient, and the slides were stained by hematoxylin and eosin (H and E) method. Then 4 µ sections were prepared for staining with H and E and also for immunohistochemical (IHC) (Ki-67, ER, PR, p53, and HER2) staining. ER and PR positivity was defined as ≥10% positive tumor cells with nuclear staining. The HER2 positive was defined as either HER2 gene amplification by fluorescent in situ hybridization (FISH) or scored as 3+ by IHC. In case of HER2 (2+), FISH was performed to determine HER2 positivity. The age, tumor size, lymph node involvement, histological grade, nuclear grade, and vascular invasion were other factors that determined in a lot of patients.
In this study, hormone receptor (HR)-positive and HER2-negative tumors were classified as luminal A type; HR-positive and HER2-positive tumors (HER2 IHC: 3+ or 2+ that amplifiedbyFISH) as luminal B type; HR-negative and HER2-positive tumors as HER2 disease; and HR-negative and HER2-negative tumors as triple negative (TN) type.[8]
Statistical analysis
Statistical analyses were performed using the IBM SPSS version 19 (SPSS Inc., Chicago, IL, USA). Chi-square test was used to analyze the significance of correlation between the expression of Ki-67 and other parameters. The value P < 0.05 was considered significant.
RESULTS
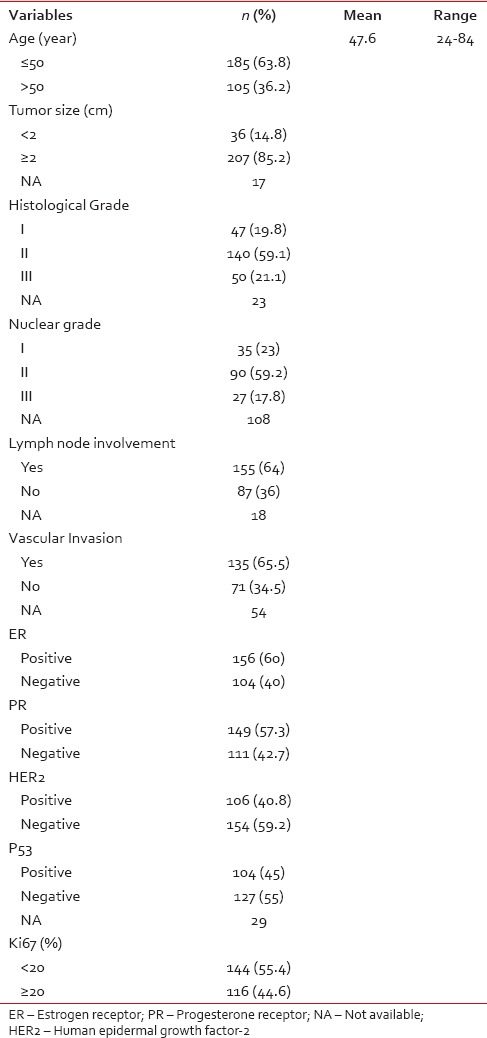
The mean age at diagnosis was 47.6 years (range, 24-84 years) with 100% female. Of 243 patients that tumor size was determined for them, 207 patients (85.2%) had tumor size ≥2 cm, and 36 patients (14.8%) had size < 2 cm and also of 237 patients, 47 patients (19.8%), 140 (59.1%), and 50 (21.1%) had histological Grades I, II, and III, respectively [Table 1]. We had nuclear grade for 152 patients that 35 patients (23%), 90 (59.2%), and 27 (17.8%) were Grades I, II, and III, respectively. Of 242 patients, 155 patients (64%) had lymph node involvement and of 206 patients, 135 patients (65.5%) had vascular invasion. Of all patients, 156 (60%), 149 (57.3%), and 106 (40.8%) were ER−, PR−, and HER2− positive, respectively. Of 231 patients, P53-positve was in 104 patients (45%). We divided Ki-67 to two groups: 144 patients (55.4%) had low Ki-67 (Ki-67 < 20%) and 116 patients (44.6%) with high Ki-67 (Ki-67 ≥20%).
Table 1
The baseline characteristics of patients with breast cancer (n = 260)
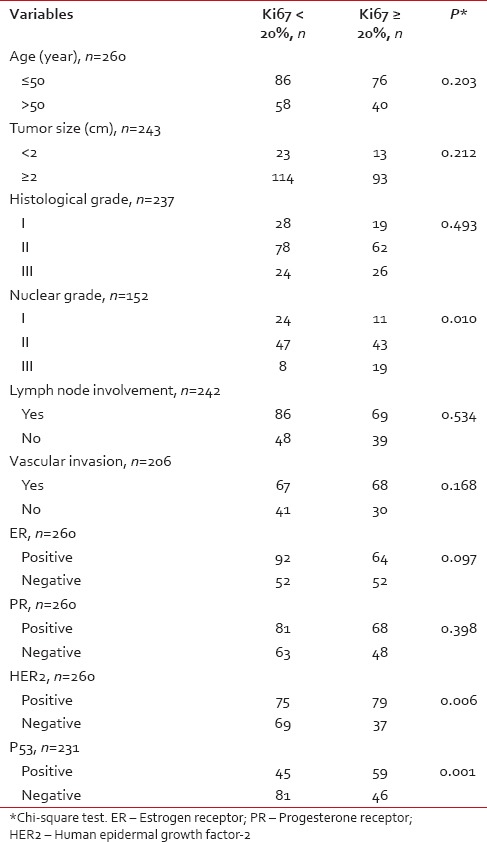
The correlation between Ki-67 with other factors in BC patients has been shown in Table 2. There is a significant correlation between Ki-67 with nuclear grade, HER2, and p53 (P < 0.05). Based on this result, more patients with Ki-67 ≥20% have a higher nuclear grade, p53-positive, and HER2-positive.
Table 2
The correlation between Ki67 with other factors in breast cancer patients
The Figure 1 shows the number of patients based on Ki-67 for types of tumor. There was correlation between Ki-67 with type of tumor (P = 0.009). Therefore, more TN patients have high Ki-67 and more patients with other subtypes have low Ki-67.
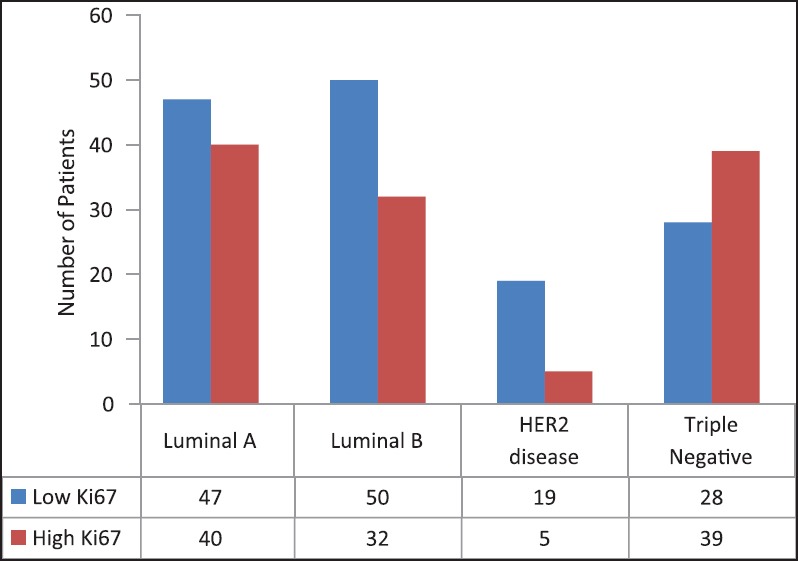
| Fig. 1 Number of patients based on Ki67 and types of tumor

| Fig. 1 Number of patients based on Ki67 and types of tumor


 PDF
PDF  Views
Views  Share
Share

