Taxane Combination Chemotherapy in Breast Cancer: Experience from a Tertiary Cancer Centre in India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 18-21
DOI: DOI: 10.4103/0971-5851.203498
Abstract
Aims:Docetaxel, Doxorubicin, Cyclophosphamide (TAC) is an intensive chemotherapy regimen; however, being highly myelosuppressive, its usage is limited in developing countries and hence merits exploration for feasibility and efficacy. Materials and Methods: This was a retrospective audit of medical records of breast cancer patients receiving TAC chemotherapy) from 2004 to 2008. Demographic details, toxicity, and outcome analysis were carried out. Results: A total of 133 patients (126 in [neo] adjuvant and 7 in metastatic setting) received TAC chemotherapy. The median age was 45 (21–67) years; 31% had coexisting diabetes and 12% hypertension. The delivered dose intensity was 94%. Discontinuation rate was 21/133 (15.8%) and the most common reason was hematological toxicity. There were 43 (32%) cases of febrile neutropenia and 2 (1.5%) Grade III thrombocytopenia with 3 (2%) toxic deaths. Grade III gastrointestinal toxicity (diarrhea) occurred in 35 (26%) and cardiac toxicity (congestive cardiac failure) in 2 (1.5%) patients. On univariate analysis, none of the variables (baseline serum albumin, hemoglobin, disease stage, or age) was found significant for chemotoxicity. At a median follow-up of 27 months (0.13–71.30 months), the estimated median disease-free survival (DFS) was 52 months in locally advanced group; however, the early breast cancer cohort has not reached to median DFS. Conclusions: TAC is an effective regimen but has significant toxicity despite the use of primary prophylactic Granulocyte Colony-Stimulating-Factor (G-GSF), including a small possibility of death. It can be considered “practically feasible” regimen in the adjuvant setting in carefully selected, fit patients.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aims:
Docetaxel, Doxorubicin, Cyclophosphamide (TAC) is an intensive chemotherapy regimen; however, being highly myelosuppressive, its usage is limited in developing countries and hence merits exploration for feasibility and efficacy.
Materials and Methods:
This was a retrospective audit of medical records of breast cancer patients receiving TAC chemotherapy) from 2004 to 2008. Demographic details, toxicity, and outcome analysis were carried out.
Results:
A total of 133 patients (126 in [neo] adjuvant and 7 in metastatic setting) received TAC chemotherapy. The median age was 45 (21–67) years; 31% had coexisting diabetes and 12% hypertension. The delivered dose intensity was 94%. Discontinuation rate was 21/133 (15.8%) and the most common reason was hematological toxicity. There were 43 (32%) cases of febrile neutropenia and 2 (1.5%) Grade III thrombocytopenia with 3 (2%) toxic deaths. Grade III gastrointestinal toxicity (diarrhea) occurred in 35 (26%) and cardiac toxicity (congestive cardiac failure) in 2 (1.5%) patients. On univariate analysis, none of the variables (baseline serum albumin, hemoglobin, disease stage, or age) was found significant for chemotoxicity. At a median follow-up of 27 months (0.13–71.30 months), the estimated median disease-free survival (DFS) was 52 months in locally advanced group; however, the early breast cancer cohort has not reached to median DFS.
Conclusions:
TAC is an effective regimen but has significant toxicity despite the use of primary prophylactic Granulocyte Colony-Stimulating-Factor (G-GSF), including a small possibility of death. It can be considered “practically feasible” regimen in the adjuvant setting in carefully selected, fit patients.
Introduction
India has been experiencing an unprecedented rise in the number of women with breast cancer, and over the last one decade, it has overtaken cervical cancer as the leading killer among women.[1] Breast cancer affects a relatively younger patient cohort (peak age of 45–49 years) in India as compared to Western standards (61 years in the USA).[2] The impact of such a grave diagnosis at such young age of peak productivity is immense.[3] Multidisciplinary, multimodality team approach has led to the real progress that we have attained over the past few decades, and it should be a source of pride and sense of achievement. Chemotherapy has been the cornerstone in the management at all stages in the natural history of disease and has led to quantum leaps unlike incremental baby steps.
In the adjuvant setting, since the landmark introduction of cyclophosphamide, methotrexate, fluorouracil (5FU) regimen by Bonadonna, anthracyclines and taxanes have been the most effective addition into our therapeutic armamentarium.[4,5] These two agents can be administered either sequentially or concurrently. However, in accordance with the Goldie-Coldman hypothesis, concurrent administration of chemotherapeutic agents as in Docetaxel, Doxorubicin, Cyclophosphamide (TAC) regimen has the theoretical advantage of overcoming drug resistance, which could translate into possible decrease in recurrence rates, which further leads to improved survival. The TAC regimen in comparison with 5FU, doxorubicin, and cyclophosphamide (FAC) regimen in node-positive breast cancer in the adjuvant setting leads to a 30% reduction in the risk of death (P = 0.008).[6] In neoadjuvant setting, TAC chemotherapy produced a 10% pathologic complete response.[7] However, the benefit of TAC may be countered by the increased incidence of hematological toxicity seen in the Western studies.[6,8,9,10]
We have become wiser from our experience with many agents which had acceptable toxicity profile in Western population while they proved too toxic in Indian studies. Such pharmacogenetic and pharmacoethnic variations[11] have been seen with 5FU, doxorubicin, cyclophosphamide, vincristine, and docetaxel. TAC regimen has three agents with proven pharmacoethnic diversity in previous studies. Hence, we decided that it would be prudent to audit and review our own institutional data regarding feasibility, safety, and efficacy profile of our patients who received TAC chemotherapy in carcinoma breast.
Materials and Methods
This is a retrospective audit of a cohort of breast cancer patients treated at our institute from 2004 to 2008 with TAC regimen in neoadjuvant, adjuvant, or first-line metastatic setting. Data were retrieved from case files and electronic medical records system at our institute. Patients regarding baseline patient demographics, disease-related variables (stage, presence of metastasis), treatment–response evaluation, and outcome analysis were carried out. The pathological complete response rates were captured, wherein TAC was used in neoadjuvant chemotherapy. Efficacy of TAC in terms of disease-free survival (DFS) in nonmetastatic setting and progression-free survival in metastatic setting was noted. The DFS was defined from date of registration till date of recurrence or progression.
All hematological and nonhematological toxicity and mortality due to toxicity were captured. Toxicity was graded in accordance with the Common Terminology Criteria for Adverse Events version 4.02.[12]
The statistical analysis was done with SPSS for Windows, Version 16.0, SPSS Inc., Chicago. The descriptive analysis was done. The Kaplan–Meir survival curves were used for estimation of DFS. The logistic regression analysis was used to detect factors predicting toxicity. The factors studied were age, baseline hemoglobin (Hb), albumin (Alb), and stage of the disease.
Results
Out of a total 200 patients offered TAC chemotherapy, 133 patients were evaluable (case records available) who had taken at least one cycle of TAC and were included in this analysis.
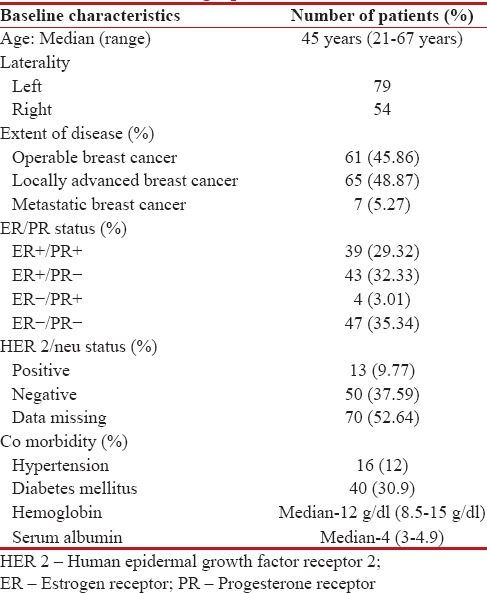
The baseline demographic profile of our patient cohort is depicted in Table 1. The mean age of patients was 45 (range of 21–67) years. Staging evaluation revealed operable disease in 46%, locally advanced breast cancer (LABC) in 49%, and metastatic disease in 5%. Estrogen receptor positivity was seen in 62% and human epidermal growth factor receptor 2 positivity in 10% of patients. Mean Hb at baseline was 12 g/dl and mean Alb was 4 g/dl.
Table 1
Baseline patient and tumor-related demographic factors
 |
| The numbers of cycles of chemotherapy planned were 798. The numbers of cycles delivered were 752; the most common reason for failure to deliver planned chemotherapy being hematological toxicity, followed by progression of disease in patients with metastatic disease. The chemotherapy could not be completed in 21 (15.78%) patients.
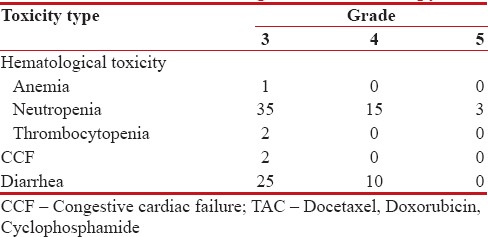
Myelosuppression was the major toxicity. There were documented infectious episodes in 77 patients (57.5%), of which 43 (32.33%) were febrile neutropenia (FN) and 3 toxic deaths. These three patients had FN associated with sepsis, had a prolonged Intensive Care Unit stay, and ultimately succumbed. Grade III thrombocytopenia was in 2 (1.5%) patients.
There were 35 (26%) Grade III/IV diarrheal episodes, and Grade II cardiac toxicity was noticed in 2 (1.5%) patients. In univariate analysis, no correlation with tested variables was found in relation to toxic events. The toxicity profile is shown in Table 2.
Table 2
Toxicities during TAC chemotherapy
|
At the median follow-up period of 27 months, the estimated median DFS was 52 months in locally advanced group; however, the early breast cancer cohort has not reached to median DFS. The 5-year estimated DFS in early breast cancer was 82% and in LABC subgroup was 52%. The estimated median DFS, Kaplan–Meir graphs in accordance with the extent of disease (elderly advanced breast cancer and LABC) are shown in Figure 1.
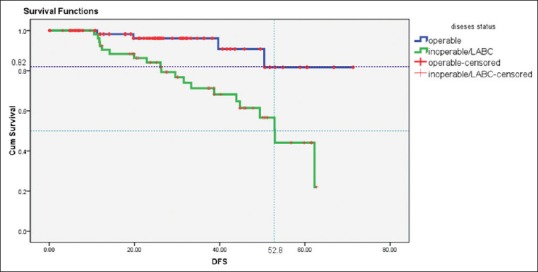
| Figure 1:Survival curves depicting disease-free survival of operable and locally advanced breast cancer cohort
Since the advent of adjuvant chemotherapy in breast cancer, efforts have been put for evolving further to improve the disease outcomes. The addition of taxanes to anthracycline-based regimens has led to an absolute 5-year risk reduction of 5% for DFS and 3% for overall survival. The administration of taxanes in various schedules (sequential or concomitant, 3 weekly or weekly) has been the focus of multitude of recent clinical trials. The benefit of combination TAC regimen over FAC regimen was shown by Martin et al., where TAC led to a 30% reduction in the risk of death.[6] However, the survival benefit came at the expense of added toxicity particularly myelosuppression 65% Grade III/IV neutropenia with 25% FN.[6] Comparisons of the hematological and cardiac side effects of the current and other studies are shown in Table 3. It can be appreciated from Table 3 that the extent of hematological side effects seen in our cohort of patients was comparable to other studies. However, incidence of FN was less than seen in a Korean study and more in comparison to few others. The reason for higher FN could be multifactorial including 50% of locally advanced disease, in a background of coexisting poor nutritional status, comorbidities (known 31% diabetes and 12% hypertension), and much more with masked coexisting diseases. With lack of support systems and social and financial constraints, these factors are contributory in noncompliant behavior of the patients in reporting adverse events and comply with the treatment of complications with resultant severe toxicity. Furthermore, although we used primary granulocyte-colony stimulating factor (G-CSF) prophylaxis for 5–7 injections (due to financial reasons), it is less than that used in many other studies.
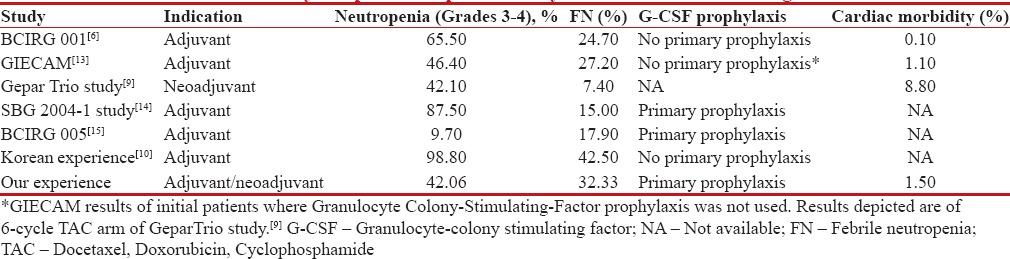
| Figure 3:
Further, there is literature evidence of variable toxicity potential in Western and Asian population. Hor et al. have shown that Indians and Chinese experience a higher degree of neutropenia compared to their other Asian counterparts when treated with doxorubicin. These pharmacogenetic/pharmacoethnic variations in treatment are very important reasons for this obvious disparity in many agents such as[16] 5FU, doxorubicin, cyclophosphamide, vincristine, and docetaxel.[11,16,17,18,19,20] TAC regimen has three agents with proven pharmacoethnic diversity in previous studies. Cardiac toxicity was 1.5% which is comparable to the reported incidence of 0.1%–8.8% in various studies, despite high comorbidity burden.
However, in light of published results of BCIRG005, the sequential use of docetaxel followed by anthracycline was comparable to the results of concurrent use as in TAC regimen.[15]
Although the follow-up is premature (27 months), the 5-year estimated DFS in early breast cancer was 82% and in LABC subgroup was 52%, which is comparable to other Western studies exploring the same regimen,[6,7,10,13,14,15] despite being nutritionally challenged and with other constraints as discussed before.
In nutritionally challenged and high tumor burden Indian patients, TAC regimen is found to be efficacious, however with significant toxicity potential despite the use of primary prophylactic G-GSF. Although toxicity profile is comparable to international standards and manageable in majority of the cases, there is small possibility of death. Hence, the choice of this regimen should always be on an individual basis. Furthermore, pharmacogenetic polymorphisms in Indian population need to be explored in future studies.
TAC is indeed a “practically feasible” and “oncologically promising” regimen in (neo) adjuvant therapy of breast cancer in the developing world in carefully selected, fit patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Agarwal G, Ramakant P. Breast cancer care in India: The current scenario and the challenges for the future. Breast Care (Basel) 2008;3:21-7.
- Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 2010;34:2308-24.
- Okonkwo QL, Draisma G, der Kinderen A, Brown ML, de Koning HJ. Breast cancer screening policies in developing countries: A cost-effectiveness analysis for India. J Natl Cancer Inst 2008;100:1290-300.
- Campone M, Fumoleau P, Bourbouloux E, Kerbrat P, Roché H. Taxanes in adjuvant breast cancer setting: Which standard in Europe? Crit Rev Oncol Hematol 2005;55:167-75.
- De Laurentiis M, Cancello G, D'Agostino D, Giuliano M, Giordano A, Montagna E, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J Clin Oncol 2008;26:44-53.
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352:2302-13.
- von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: Phase III randomized GeparTrio study. J Natl Cancer Inst 2008;100:552-62.
- Martín M, Lluch A, Seguí MA, Ruiz A, Ramos M, Adrover E, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): Impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol 2006;17:1205-12.
- von Minckwitz G, Kümmel S, du Bois A, Eiermann W, Eidtmann H, Gerber B, et al. Pegfilgrastim +/- ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 2008;19:292-8.
- HD Woo HK, Lee JH, Kim HM, Han SW, Kim SY, Lim CW, et al. Toxicity and Tolerability Study of Adjuvant TAC Regimen Chemotherapy in Korean Patients with Breast Cancer. J Breast Cancer 2011;14(S):S44-9.
- O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: Ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 2009;15:4806-14.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.02 U.S. Department Of Health And Human Services National Institutes of Health National Cancer Institute.
- Martín M1, Lluch A, Seguí MA, Ruiz A, Ramos M, Adrover E, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol. 2006;17:1205-12.
- Margolin S, Bengtsson NO, Carlsson L, Edlund P, Hellstrøm M, Karlsson P, et al. Arandomised feasibility/phase II study (SBG 2004-1) with dose-dense/tailored epirubicin, cyclophoshamide (EC) followed by docetaxel (T) or fixed dosed dose-dense EC/T versus T, doxorubicin and C (TAC) in node-positive breast cancer. Acta Oncol 2011;50:35-41.
- Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 2011;29:3877-84.
- Hor SY, Lee SC, Wong CI, Lim YW, Lim RC, Wang LZ, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J 2008;8:139-46.
- Lal S, Wong ZW, Jada SR, Xiang X, Chen Shu X, Ang PC, et al. Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics 2007;8:567-75.
- Sandanaraj E, Lal S, Selvarajan V, Ooi LL, Wong ZW, Wong NS, et al. PXR pharmacogenetics: Association of haplotypes with hepatic CYP3A4 and ABCB1 messenger RNA expression and doxorubicin clearance in Asian breast cancer patients. Clin Cancer Res 2008;14:7116-26.
- Tan SH, Lee SC, Goh BC, Wong J. Pharmacogenetics in breast cancer therapy. Clin Cancer Res 2008;14:8027-41.
- Afsar NA, Ufer M, Haenisch S, Remmler C, Mateen A, Usman A, et al. Relationship of drug metabolizing enzyme genotype to plasma levels as well as myelotoxicity of cyclophosphamide in breast cancer patients. Eur J Clin Pharmacol 2012;68:389-95.

| Figure 1:Survival curves depicting disease-free survival of operable and locally advanced breast cancer cohort
References
- Agarwal G, Ramakant P. Breast cancer care in India: The current scenario and the challenges for the future. Breast Care (Basel) 2008;3:21-7.
- Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 2010;34:2308-24.
- Okonkwo QL, Draisma G, der Kinderen A, Brown ML, de Koning HJ. Breast cancer screening policies in developing countries: A cost-effectiveness analysis for India. J Natl Cancer Inst 2008;100:1290-300.
- Campone M, Fumoleau P, Bourbouloux E, Kerbrat P, Roché H. Taxanes in adjuvant breast cancer setting: Which standard in Europe? Crit Rev Oncol Hematol 2005;55:167-75.
- De Laurentiis M, Cancello G, D'Agostino D, Giuliano M, Giordano A, Montagna E, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J Clin Oncol 2008;26:44-53.
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352:2302-13.
- von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: Phase III randomized GeparTrio study. J Natl Cancer Inst 2008;100:552-62.
- Martín M, Lluch A, Seguí MA, Ruiz A, Ramos M, Adrover E, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): Impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol 2006;17:1205-12.
- von Minckwitz G, Kümmel S, du Bois A, Eiermann W, Eidtmann H, Gerber B, et al. Pegfilgrastim +/- ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 2008;19:292-8.
- HD Woo HK, Lee JH, Kim HM, Han SW, Kim SY, Lim CW, et al. Toxicity and Tolerability Study of Adjuvant TAC Regimen Chemotherapy in Korean Patients with Breast Cancer. J Breast Cancer 2011;14(S):S44-9.
- O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: Ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 2009;15:4806-14.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.02 U.S. Department Of Health And Human Services National Institutes of Health National Cancer Institute.
- Martín M1, Lluch A, Seguí MA, Ruiz A, Ramos M, Adrover E, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol. 2006;17:1205-12.
- Margolin S, Bengtsson NO, Carlsson L, Edlund P, Hellstrøm M, Karlsson P, et al. Arandomised feasibility/phase II study (SBG 2004-1) with dose-dense/tailored epirubicin, cyclophoshamide (EC) followed by docetaxel (T) or fixed dosed dose-dense EC/T versus T, doxorubicin and C (TAC) in node-positive breast cancer. Acta Oncol 2011;50:35-41.
- Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 2011;29:3877-84.
- Hor SY, Lee SC, Wong CI, Lim YW, Lim RC, Wang LZ, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J 2008;8:139-46.
- Lal S, Wong ZW, Jada SR, Xiang X, Chen Shu X, Ang PC, et al. Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics 2007;8:567-75.
- Sandanaraj E, Lal S, Selvarajan V, Ooi LL, Wong ZW, Wong NS, et al. PXR pharmacogenetics: Association of haplotypes with hepatic CYP3A4 and ABCB1 messenger RNA expression and doxorubicin clearance in Asian breast cancer patients. Clin Cancer Res 2008;14:7116-26.
- Tan SH, Lee SC, Goh BC, Wong J. Pharmacogenetics in breast cancer therapy. Clin Cancer Res 2008;14:8027-41.
- Afsar NA, Ufer M, Haenisch S, Remmler C, Mateen A, Usman A, et al. Relationship of drug metabolizing enzyme genotype to plasma levels as well as myelotoxicity of cyclophosphamide in breast cancer patients. Eur J Clin Pharmacol 2012;68:389-95.


 PDF
PDF  Views
Views  Share
Share

