Symptom Burden and Quality of Life Issues among Patients of Chronic Myeloid Leukemia on Long-term Imatinib Therapy
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 165-168
DOI: DOI: 10.4103/ijmpo.ijmpo_160_16
Abstract
Background: Tyrosine kinase inhibitors such as imatinib have improved survival in chronic myeloid leukemia (CML). Imatinib can cause chronic side effects which are not considered serious but can impact the quality of life (QoL) of the patient. Methods: The results of a detailed symptom burden analysis and its impact on QoL scores in a cohort of patients on long-term imatinib is presented in this study. Symptom burden was assessed using the M. D. Anderson Symptom Inventory specific for CML patients. An indigenously developed QoL questionnaire (Cancer Institute Quality of Life II) was administered simultaneously. Results: Of 221 patients of CML (M:F = 133:88; median age: 39 years [18–65], median duration of imatinib: 4 years), QoL scores were high in 46%, average in 39%, and low in 14%. QoL scores were negatively correlated with general symptoms (r = −0.612, P < 0 class="i" xss=removed>r = −0.513, P < 0 class="i" xss=removed>r = −0.596, P < 0>
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Tyrosine kinase inhibitors such as imatinib have improved survival in chronic myeloid leukemia (CML). Imatinib can cause chronic side effects which are not considered serious but can impact the quality of life (QoL) of the patient.
Methods:
The results of a detailed symptom burden analysis and its impact on QoL scores in a cohort of patients on long-term imatinib is presented in this study. Symptom burden was assessed using the M. D. Anderson Symptom Inventory specific for CML patients. An indigenously developed QoL questionnaire (Cancer Institute Quality of Life II) was administered simultaneously.
Results:
Of 221 patients of CML (M:F = 133:88; median age: 39 years [18–65], median duration of imatinib: 4 years), QoL scores were high in 46%, average in 39%, and low in 14%. QoL scores were negatively correlated with general symptoms (r = −0.612, P < 0 xss=removed>r = −0.513, P < 0 xss=removed>r = −0.596, P < 0>
Conclusions:
Significant impairment of QoL was noted among patients with CML primarily due to the burden of symptom related to side effects of imatinib. This issue must be addressed both in the clinic as well as in all studies of CML.
Introduction
Chronic myeloid leukemia (CML) accounts for one-fifth of all leukemias and has an incidence of 1.0–1.5/100,000.[1,2] Though survival in CML has greatly improved with the use of oral tyrosine kinase inhibitors such as imatinib, best outcomes require strict adherence to therapy.[3,4] Poor adherence to therapy in CML is strongly associated with lower quality of life (QoL) which in turn may be affected by multiple factors, including symptoms caused by side effects of imatinib.[5,6,7,8,9,10,11,12,13,14] These issues are often neglected in patients on long-term therapy with imatinib.[8,15,16]
We had shown in our earlier publication that poor QoL scores (assessed by the EORTC questionnaire) are strongly associated with nonadherence to therapy in a cohort of patients on long-term imatinib.[10] Additional assessments were carried out in the same cohort of patients specifically focusing on their symptom burden. Simultaneously, the usefulness of an indigenously developed QoL questionnaire was assessed for the first time in a cohort of patients with CML.[17] These results of these assessments are presented in this study.
Methods
Patient population and study design
Adult patients with chronic-phase CML (n = 221) who were on imatinib for at least 6 months were included in the study. The demographic data, adherence to therapy, its correlation with the EORTC QOL, and association with molecular responses have been described in an earlier study.[10] In this study, we describe the results of the assessments carried out to understand the symptom burden of the patients and the correlation with an indigenously developed and validated QoL tool.
Assessment of symptom burden
The M. D. Anderson Symptom Inventory specific for CML patients (MDASI-CML), a multi-symptom patient-reported outcome measure, was used for the assessment of symptom burden.[18] The inventory assesses the severity of symptoms at their worst in the last 24 h on a 0–10 numerical rating scale with 0 being “not present” and 10 being “as bad as you can imagine.” In addition to 13 items related to symptoms found in highest frequency and/or severity in patients’ cancers, seven CML-specific symptoms are included in MDASI-CML. The tool also measures the interference of symptoms with six daily activities: general activity, mood, work, relations with others, walking, and enjoyment of life. Interference is rated on a 0–10 numerical rating scale, 0 being “did not interfere” and 10 being “interfered completely.” As per MDASI-CML, for the purpose of analysis, a prorated total score is calculated when patients score was at least 7 of the 13 items using the following formula: (sum of items answered) ×13/number of items answered.
Assessment of quality of life using an indigenously developed tool
QoL among patients of CML was measured by the Cancer institute Quality of Life II questionnaire.[17] This 41-item tool measured 11 dimensions of QoL which included general well-being, physical well-being, psychological well-being, interpersonal relationship, sexual and personal well-being, cognitive well-being, optimism, economical well-being, informational support, patient–physician relationship, and body image. Of the 41 items, 39 items were in Likert 4-point scale and the remaining two items were in 10-point semantic scale, ranging from 1 to 10 with two extremes, namely very poor and excellent.
Scoring of the tool instructs both direct scoring and reverse scoring with a minimum score of 42 and a maximum score of 180. Higher score indicates better QoL (score < 99 xss=removed xss=removed xss=removed> 165 = very high QoL).
Statistical analysis
For the ease of analysis, the five levels of QOL were re-organized as three levels (low combining low and very low scores, average and high combining high and very high scores).[17] Association of sociodemographic parameters and level of QoL was analyzed using Chi-square test. A bivariate correlation was used to analyze the association between QoL and symptom burden. SPSS ver 17.0 (SPSS Inc., Chicago) was used for the analysis.
Results
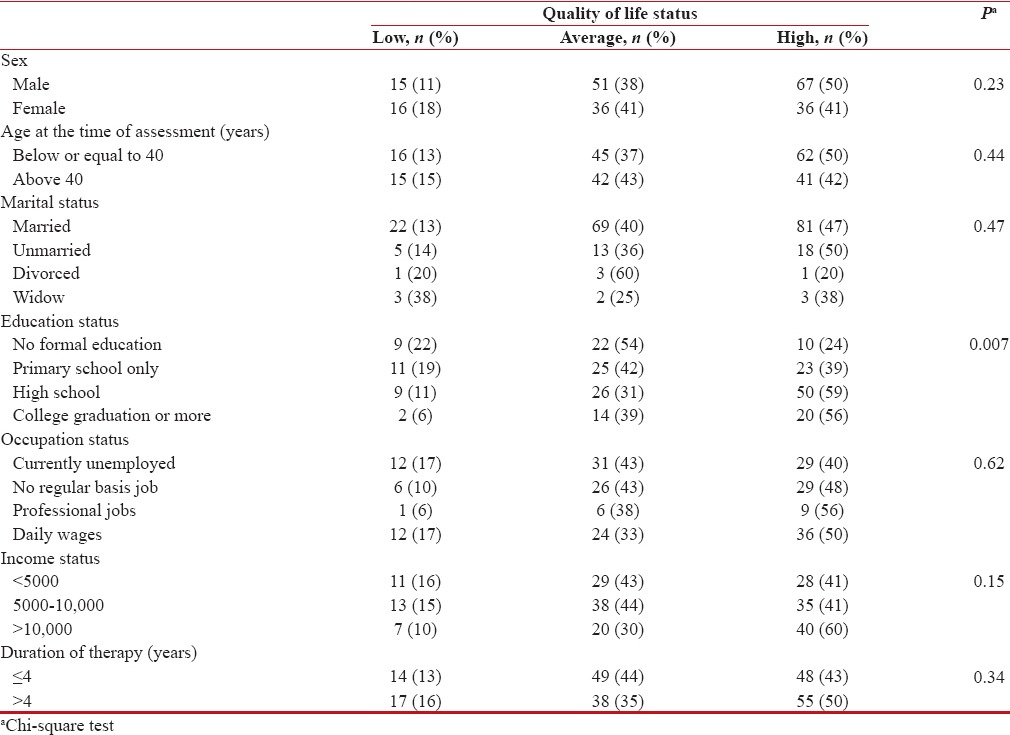
The baseline demography of the patients is shown in Table 1. Among the 221 patients (M:F = 133:88; median age: 39 years [18–65]) of CML (median duration of treatment: 4 [1–13] years), more than half (55%) had high school education, and of them, 40% were employed. Female patients who were homemakers were included in the “currently unemployed” group. Distribution of income status on monthly basis (international normalized ratio) was <5000>10,000 (31%).
Table 1
Demographic characteristics (N==221)a

|
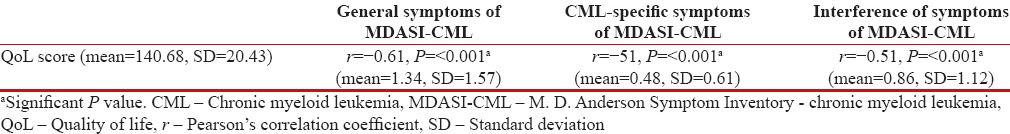
QoL scores were high in 46%, average in 39%, and low in 14%. Other than the educational status, none of the demographic baseline characteristics predicted QoL levels [Table 2]. A significant association was noted between symptom scores and QoL. On bivariate correlation, QoL scores were negatively correlated with general symptoms (r = −0.61, P < 0>r = −0.51, P < 0>r = −0.59, P < 0>Table 3 and Figure 1].
Table 2
Association of quality of life with demographic characteristics
|
Table 3
Correlation of the Cancer Institute Quality of Life questionnaire with general symptoms, chronic myeloid leukemia-specific symptoms, and interference of symptoms of M. D. Anderson Symptom Inventory - chronic myeloid leukemia
|
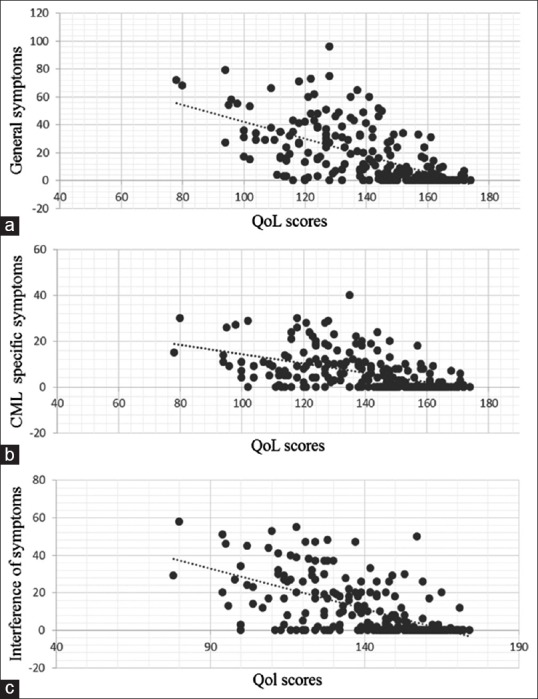
| Figure 1:The correlation between the quality of life scores as per the Chronic Illness Quality of Life questionnaire and the symptom score assessed by the M. D. Anderson Symptom Inventory. The various components of the symptom score-symptom severity (a), chronic myeloid leukemia-specific symptoms (b), and interference of symptoms (c) are shown. The lowest and highest possible scores for quality of life were 40 and 190, respectively
Discussion
In our earlier study, we premised that side effects of imatinib lead to poor QoL and this causes patients to miss pills (nonadherence) which compromise the outcomes of therapy.[10] In a more detailed analysis specifically focused on the symptom burden, we demonstrate the direct effect of high symptom scores on the QoL of the patients. We also demonstrate the usefulness of an indigenously developed QoL questionnaire in a population of patients with long-term cancer treatment. Significantly, more than half the patients had low or average QoL, a major issue which is not addressed by clinicians during their regular follow-up of patients.
Earlier studies reported that adverse events of patients with CML result in treatment discontinuation and thereby negatively affect treatment efficacy and QoL.[9,14,15,16,18,19] On the development of MDASI-CML questionnaire, its authors pointed out that prolonged period of moderate-to-severe symptoms would definitely interfere with patients’ functional status and QoL outcome for which they suggested routine symptom monitoring and management.[12] Necessity of providing education, support, and assistance to patients for management of adverse events to optimize outcomes has been highlighted elsewhere.[15]
Although these issues have been highlighted by other studies, this is one of the first studies from India demonstrating the problems caused by symptom burden in patients on long-term imatinib. We were able to validate the usefulness of an indigenously developed QoL tool in a population of patients with CML. We have found that using the indigenous tool is much easier and culturally acceptable than the EORTC QLQ. Though imatinib has greatly increased survival in patients with CML, it also imposes a burden which is not obvious and often ignored in a busy clinic. We strongly recommend every doctor managing patients of CML to actively seek details of side effects that patient may be harboring and identify ways to remove or at least reduce these symptoms. These efforts could greatly enhance patient adherence to therapy and in turn outcomes of therapy.
Financial support and sponsorship
The study protocol was supported by Cancer Institute (WIA) funds. No grant number is applicable.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank Dr. Charles Cleeland and others of MD Anderson cancer center for providing MDASI-CML tool and its scoring key
References
- Goldman JM. Chronic myeloid leukemia in India. Indian J Med Paediatr Oncol 2013;34:147-8.
- Mauro MJ, Davis C, Zyczynski T, Khoury HJ. The role of observational studies in optimizing the clinical management of chronic myeloid leukemia. Ther Adv Hematol 2015;6:3-14.
- Kantarjian H, O'Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: A single-institution historical experience. Blood 2012;119:1981-7.
- Saussele S, Krauss MP, Hehlmann R, Lauseker M, Proetel U, Kalmanti L, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: Results of the randomized CML study IV. Blood 2015;126:42-9.
- Ganesan P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011;86:471-4.
- Noens L, Hensen M, Kucmin-Bemelmans I, Lofgren C, Gilloteau I, Vrijens B. Measurement of adherence to BCR-ABL inhibitor therapy in chronic myeloid leukemia: Current situation and future challenges. Haematologica 2014;99:437-47.
- Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010;28:2381-8.
- Efficace F, Baccarani M, Breccia M, Alimena G, Rosti G, Cottone F, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 2011;118:4554-60.
- Efficace F, Baccarani M, Breccia M, Cottone F, Alimena G, Deliliers GL, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 2013;27:1511-9.
- Unnikrishnan R, Veeraiah S, Mani S, Rajendranath R, Rajaraman S, Vidhubala Elangovan GS, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk 2016;16:366-71.e3.
- Baccarani M, Efficace F, Rosti G. Moving towards patient-centered decision-making in chronic myeloid leukemia: Assessment of quality of life and symptom burden. Haematologica 2014;99:205-8.
- Williams LA, Garcia Gonzalez AG, Ault P, Mendoza TR, Sailors ML, Williams JL, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 2013;122:641-7.
- Hirji I, Gupta S, Goren A, Chirovsky DR, Moadel AB, Olavarria E, et al. Chronic myeloid leukemia (CML): Association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient's perspective. Health Qual Life Outcomes 2013;11:167.
- Cella D, Nowinski CJ, Frankfurt O. The impact of symptom burden on patient quality of life in chronic myeloid leukemia. Oncology 2014;87:133-47.
- Cornelison M, Jabbour EJ, Welch MA. Managing side effects of tyrosine kinase inhibitor therapy to optimize adherence in patients with chronic myeloid leukemia: The role of the midlevel practitioner. J Support Oncol 2012;10:14-24.
- Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: Definitions and clinical implications. Cancer 2011;117:688-97.
- Vidhubala E, Kannan RR, Mani SC, Karthikesh K, Muthuvel R, Surendran V, et al. Validation of quality of life questionnaire for patients with cancer – Indian scenario. Indian J Cancer 2005;42:138-44.
- Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634-46.
- Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 2011;103:553-61.

| Figure 1:The correlation between the quality of life scores as per the Chronic Illness Quality of Life questionnaire and the symptom score assessed by the M. D. Anderson Symptom Inventory. The various components of the symptom score-symptom severity (a), chronic myeloid leukemia-specific symptoms (b), and interference of symptoms (c) are shown. The lowest and highest possible scores for quality of life were 40 and 190, respectively
References
- Goldman JM. Chronic myeloid leukemia in India. Indian J Med Paediatr Oncol 2013;34:147-8.
- Mauro MJ, Davis C, Zyczynski T, Khoury HJ. The role of observational studies in optimizing the clinical management of chronic myeloid leukemia. Ther Adv Hematol 2015;6:3-14.
- Kantarjian H, O'Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: A single-institution historical experience. Blood 2012;119:1981-7.
- Saussele S, Krauss MP, Hehlmann R, Lauseker M, Proetel U, Kalmanti L, et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: Results of the randomized CML study IV. Blood 2015;126:42-9.
- Ganesan P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 2011;86:471-4.
- Noens L, Hensen M, Kucmin-Bemelmans I, Lofgren C, Gilloteau I, Vrijens B. Measurement of adherence to BCR-ABL inhibitor therapy in chronic myeloid leukemia: Current situation and future challenges. Haematologica 2014;99:437-47.
- Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010;28:2381-8.
- Efficace F, Baccarani M, Breccia M, Alimena G, Rosti G, Cottone F, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 2011;118:4554-60.
- Efficace F, Baccarani M, Breccia M, Cottone F, Alimena G, Deliliers GL, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 2013;27:1511-9.
- Unnikrishnan R, Veeraiah S, Mani S, Rajendranath R, Rajaraman S, Vidhubala Elangovan GS, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk 2016;16:366-71.e3.
- Baccarani M, Efficace F, Rosti G. Moving towards patient-centered decision-making in chronic myeloid leukemia: Assessment of quality of life and symptom burden. Haematologica 2014;99:205-8.
- Williams LA, Garcia Gonzalez AG, Ault P, Mendoza TR, Sailors ML, Williams JL, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 2013;122:641-7.
- Hirji I, Gupta S, Goren A, Chirovsky DR, Moadel AB, Olavarria E, et al. Chronic myeloid leukemia (CML): Association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient's perspective. Health Qual Life Outcomes 2013;11:167.
- Cella D, Nowinski CJ, Frankfurt O. The impact of symptom burden on patient quality of life in chronic myeloid leukemia. Oncology 2014;87:133-47.
- Cornelison M, Jabbour EJ, Welch MA. Managing side effects of tyrosine kinase inhibitor therapy to optimize adherence in patients with chronic myeloid leukemia: The role of the midlevel practitioner. J Support Oncol 2012;10:14-24.
- Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: Definitions and clinical implications. Cancer 2011;117:688-97.
- Vidhubala E, Kannan RR, Mani SC, Karthikesh K, Muthuvel R, Surendran V, et al. Validation of quality of life questionnaire for patients with cancer – Indian scenario. Indian J Cancer 2005;42:138-44.
- Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634-46.
- Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 2011;103:553-61.


 PDF
PDF  Views
Views  Share
Share

