Stem cell transplant: An experience from eastern India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(04): 203-209
DOI: DOI: 10.4103/0971-5851.107078
Abstract
Background: Hematopoietic stem cell transplant using human leukocyte antigen (HLA)-matched sibling or unrelated bone marrow, or related or unrelated cord blood has been performed successfully to treat patients with different types of hematological malignancies, genetic disorders and hereditary immune deficiencies. Since 1983, stem cell transplantation has been carried out in different institutes of India. But, till then, no transplantation was performed in eastern India. Materials and Methods: Our present study is reporting for the first time stem cell transplantation in eastern India. From August 2000 to June 2011 (with a 3-year gap for up-gradation), we have performed a total of 22 transplants. Thirteen patients (M:F:9:4) with indications of aplastic anemia, thalassaemia, acute myeloid leukemia and chronic myeloid leukemia underwent allogenic transplant, whereas autologous transplant was performed for nine patients (M:F:2:1) of multiple myeloma, Hodgkin′s and non-Hodgkin′s lymphoma and neuroblastoma. The median age of the patients was 19.6 years, with a range of 5 years 8 months to 52 years. Fourteen patients received myeloablative conditioning regime whereas eight patients received immunosuppressive and less myeloablative protocol. Sources of stem cells in case of allogenic transplant are bone marrow and related or unrelated umbilical cord blood and in case of autologous transplant, these are peripheral blood stem cells or self-bone marrow. Standard prophylactic medication was followed prior to transplants. Results: A disease-free survival of 68.18% and overall survival of 86.3% were seen at the median follow-up period of 4.6 years. Common post-transplant complications were mucositis, infection, venoocclusive disease, graft versus host disease, hemorrhagic cystitis, etc. Conclusion: The use of cord blood as a source of stem cells has been proved inferior as compared with the bone marrow stem cell source in cases of thalassaemia in our institute and thus is not recommended for thalassaemia. But, it has been proved to be a very useful and effective stem cell source (both related and unrelated cord blood) in cases of aplastic anemia and other immunological disorders.
Keywords
Aplastic anemia - bone marrow transplantation - cord blood transplantation - eastern Indian data - stem cell transplantation - thalassaemiaPublication History
Article published online:
20 July 2021
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Hematopoietic stem cell transplant using human leukocyte antigen (HLA)-matched sibling or unrelated bone marrow, or related or unrelated cord blood has been performed successfully to treat patients with different types of hematological malignancies, genetic disorders and hereditary immune deficiencies. Since 1983, stem cell transplantation has been carried out in different institutes of India. But, till then, no transplantation was performed in eastern India.
Materials and Methods:
Our present study is reporting for the first time stem cell transplantation in eastern India. From August 2000 to June 2011 (with a 3-year gap for up-gradation), we have performed a total of 22 transplants. Thirteen patients (M:F:9:4) with indications of aplastic anemia, thalassaemia, acute myeloid leukemia and chronic myeloid leukemia underwent allogenic transplant, whereas autologous transplant was performed for nine patients (M:F:2:1) of multiple myeloma, Hodgkin's and non-Hodgkin's lymphoma and neuroblastoma. The median age of the patients was 19.6 years, with a range of 5 years 8 months to 52 years. Fourteen patients received myeloablative conditioning regime whereas eight patients received immunosuppressive and less myeloablative protocol. Sources of stem cells in case of allogenic transplant are bone marrow and related or unrelated umbilical cord blood and in case of autologous transplant, these are peripheral blood stem cells or self-bone marrow. Standard prophylactic medication was followed prior to transplants.
Results:
A disease-free survival of 68.18% and overall survival of 86.3% were seen at the median follow-up period of 4.6 years. Common post-transplant complications were mucositis, infection, venoocclusive disease, graft versus host disease, hemorrhagic cystitis, etc.
Conclusion:
The use of cord blood as a source of stem cells has been proved inferior as compared with the bone marrow stem cell source in cases of thalassaemia in our institute and thus is not recommended for thalassaemia. But, it has been proved to be a very useful and effective stem cell source (both related and unrelated cord blood) in cases of aplastic anemia and other immunological disorders.
INTRODUCTION
In order to treat patients with high-risk or relapsed hematologic malignancies, hereditary immune deficiencies, metabolic disorders and some other life-threatening non-malignant ailments like thalassaemia, human leukocyte antigen (HLA)-matched or unrelated hematopoietic stem cell transplantation has been used successfully as a life-saving procedure for a long period. In September 2006, data from six transplant centers in India were collected and it was found that a total of 1540 allogenic and autologous bone marrow transplantations (BMT) have been performed in a country of over one billion population.[1] Presently, more than 40,000 stem cell transplantations are being performed annually worldwide.[2] In India, the transplantation procedure is being practiced in the larger transplant centers, located mostly in western, northern and southern India. But, there was no transplantation center in the eastern part of India for performing transplantation before 2000 CE.
West Bengal has a population of 100 million people, with < 5% having insurance coverage. Twenty-five percent of the people belong to the higher middle class and 70% to the lower middle class with poor socioeconomic status. Therefore, only 25–30% of the people can afford transplant treatment. Thalassaemia major is highly prevalent in this part of the country. There is a total of 26,000 thalassaemia major patients, with an annual increment of 2,500. If it is expected that 1/3rd of those patients have HLA-matched sibling donors, then there is a potential cure of approximately 800 thalassemia children every year with BMT. Other patients who can be candidates of allogenic stem cell transplants are aplastic anemia, acute myeloid leukemia (AML), chronic myeloid leukemia (CML) - resistant to imatinib, storage diseases and immunodeficiency disorders. Autologous stem cell transplant can be performed in multiple myeloma, neuroblastoma and lymphoma. Considering all the indications, almost 3000 patients can be provided with stem cell therapy by the state. But, unfortunately, only 700–750 can afford transplant, either bearing their own cost or through private insurance or corporate.
In this background, it was a big challenge to set up a new non-governmental stem cell unit to cater to the patients with a low economic profile. We started our Bone Marrow and Stem Cell Transplant programme for first time in eastern India in 2000 CE, and upgraded our unit in 2007 in the Netaji Subhas Chandra Bose Cancer Research Institute (NCRI) in Kolkata [Figure 1], with a mission to minimize the cost to make the middle and lower-middle class of the society avail the transplant facility. Therefore, our main objective was to establish a cost-effective transplant center in eastern India to provide the patients of eastern India with the service of various types of hematopoietic stem cell transplantation. We have performed 22 transplants successfully till date. In the present study, we have reported the result of stem cell transplant in various indications in the NCRI.
| Fig. 1 The location of NCRI, Kolkata in the map of India
MATERIALS AND METHODS
NCRI, a charitable non-governmental trust-run institute, is a tertiary cancer center in Kolkata with hematology–oncology units. This institute has the facilities of chemotherapy and surgical oncology for the treatment of hematological disorders and solid tumors. DNB course in Medical Oncology and PhD programmes in Molecular Biology and Human Genetics are also running in the institute. The Research and Development unit runs the government-funded projects and several national and international clinical trials. Every year, almost 6000 new indoor and 15,000 new OPD patients are attending this hospital. It is a referral center for hemato–oncology and BMT patients. Patients of leukemia, thalassemia and aplastic anemia are the main patient pools for transplant.
Transplant unit
Because of the much higher level of bacterial infection and polluted environment in the city of Kolkata, it is mandatory to have a dedicated High-Efficiency Particulate Air (HEPA) filter transplant unit to prevent any kind of infections in the transplant room. Presently, we have two rooms consisting of two beds with the HEPA filter system. We have in-house pathology, serology, cytogenetics, molecular biology, apheresis and -80°C storage system facilities.
Till 2011 June, we have performed 22 transplants at the NCRI, of which pediatric patients were 10 and adults were 12. The median age of the patients was 19.6 years, with a range of 5 years 8 months to 52 years. The male to female ratio is 15:7. The distribution of cases is described in Tables Tables11 and and22.
Table 1
The distribution of cases with indications, sources of stem cells and HLA matching status in allogenic stem cell transplantation
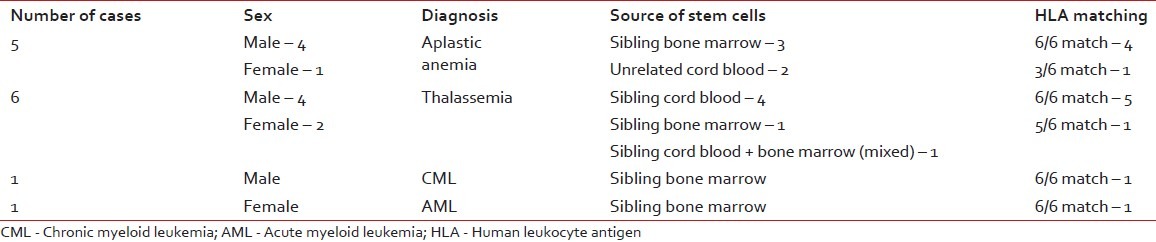
Table 2
The distribution of cases with indications and sources of stem cells in autologous stem cell transplantation
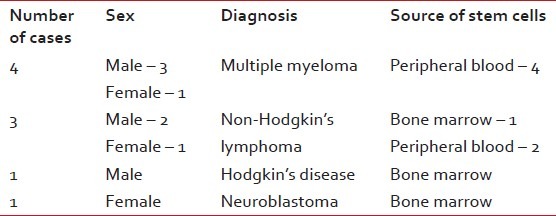
Transplant protocol conditioning
From Day -14 (14 days prior to transplant), all patients started the conditioning chemotherapy as mentioned in Table 3. Graft-versus-host-disease (GVHD) prophylaxis is mentioned in Table 4.
Table 3
The conditioning chemotherapy prior to transplants
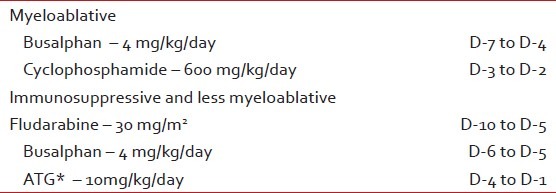
Table 4
Graft-versus-host-disease prophylaxis

Antimicrobial prophylaxis
From Day -14 (14 days prior to transplant), all patients started prophylactic medications as mentioned below in Table 5.
Table 5
Prophylactic medication administered

Stem cell collection
Bone marrow harvesting
For all cases of thalassaemia, aplastic anemia and leukemia, the bone marrow is a preferred source of stem cells. We used the Trephine Biopsy needle and collected the required volume of bone marrow (200–1000 mL as per weight of the donor) by puncturing the posterior superior iliac spine or from the posterior iliac crest. Autologous GCSF-mobilized peripheral blood or bone marrow was collected in case of multiple myeloma, lymphoma or neuroblastoma.
Umbilical cord blood collection and processing
In those cases where fully HLA-matched bone marrow donor was not available, we used matched unrelated cord blood or partially mismatched umbilical cord blood. Cord blood was collected during caesarian delivery or 3rd stage of labor from umbilical cord. After collection, it was mixed with DMSO and was stored at -80°C for short term (up to 3 months) or cryopreserved in liquid nitrogen (-192°C) for long term.
Peripheral blood stem cell collection
Allogenic or autologous GCSF-mobilized peripheral blood was collected by a cell separator machine (Amicus, Fenwell), mixed with DMSO and stored for short term at -80°C. Table 6 describes the characteristics of infused stem cell.
Table 6
The characteristics of infused stem cell among patients

RESULTS
Engraftment
When the absolute neutrophil count became >500 for three consecutive days, it was considered as WBC engraftment. The mean time for WBC engraftment was 19 days (range: 11–38 days) in our series of patients. Unsupported hemoglobin >10 mg/dL in three consecutive days was considered as hemoglobin engraftment. The mean time of hemoglobin engraftment for our patients was 38 days (range: 28–86 days). When unsupported platelet count was >50,000 for three consecutive days, it is considered as platelet engraftment. The mean time for platelet engraftment was 26 days (range: 18–57 days) in this present study. The mean time for WBC, platelet and hemoglobin engraftment are shown in Figure 2.
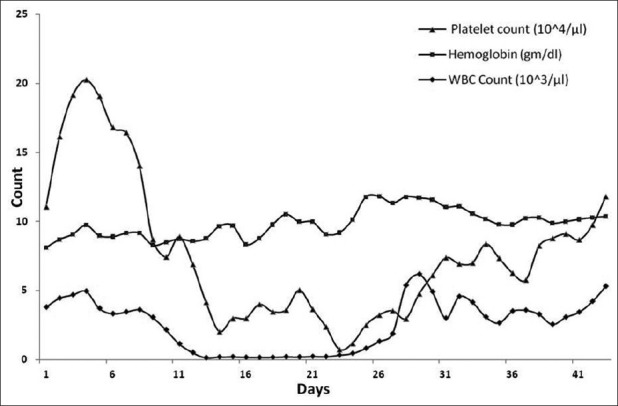
| Fig. 2 Engraftment of WBC, platelet and hemoglobin in the series of patients
Change of blood group
Engraftment and chimerism were studied by karyotyping and polymerase chain reaction for sex-mismatched transplants. In blood group-mismatched transfusion, the mean time for blood group changeover was 75 days (range: 62–118 days) in our study group.
Change of sex chromosome
The mean time for change of sex chromosome was 92 days (from 72 to 200 days) in case of sex-mismatched transplants.
Transfusion support
Irradiated blood products were given in the post-transplant period for our transplant patients. Mean single-donor platelet requirement was 4 (range: 2–10), mean packed red cell requirement was 3 (range: 1-6) and mean growth factor requirement was 12 (range: 6–27) in our group of patients. The criteria were to keep the platelet count greater than 20,000/dL in febrile patients and, in afebrile patients, the acceptable limit was 10,000/dL.
Post-transplant complications
Early complications before 100 days
The most common post-transplant complications were mucositis, infections, venoocclusive disease (VOD), GVHD and hemorrhagic cystitis.
Mucositis
One of the most common complications of BMT is mucositis. Grade I mucositis was seen in 100% patients and four (18%) developed Grade II + III mucositis.
Infection
There were 25 incidences of documented infection among 22 transplants. The distribution of infections was bacterial in 52% cases. Bacterial organisms were mainly gram –ve (80%) as compared with gram +ve (20%). The most common gram –ve organisms were E. coli, (60%), Pseudomonas (25%), Klebsiella (10%) and others (5%). The major gram +ve infection was Methicillin-resistant Staphylococcus aureous (75%). The major source of the positive culture was blood (50%), followed by urine and sputum.
VOD
Two (9.09%) of our patients developed hepatic VOD. They presented with jaundice, ascitis and weight gain. The predisposing factor was pre-transplant conditioning with Busulphan and Cyclophosphamide.
GVHD
Acute GVHD occurs generally within 100 days of transplant. Of 22 patients, four patients (18.18%) developed Grade II GVHD of skin, three patients (13.63%) developed Grade I GVHD of liver and two patients (9.09%) developed Grade II–III gut GVHD.
Hemorrhagic cystitis
It occurs due to Cyclophosphamide conditioning. Severe hemorrhagic cystitis was seen in one patient (4.54%) who was managed conservatively.
Late complications at 100 days
Chronic GVHD
It occurred after 10 days of transplant, which was seen only in two cases (9.09%) with chronic skin GVHD.
Infection
Of the total infection episodes, viral (24%) and fungal (12%) infections were seen as chronic complications. Two patients had documented tubercular infection on the 2nd and 3rd month post-transplant, and were on four-drug ATD therapies.
OUTCOMES
Overall survival
Overall survival (OS) is a term that denotes the chances of staying alive for a group of individuals suffering from a particular disease. Here, it denoted the percentage of individuals in the group who are likely to be alive after a particular duration of time (4.6 years in this study). Before upgrading our unit (2000–2004 CE), we performed nine transplants and, during the period of 2007–2011, we have done 13 transplants. As we are considering all the patients done in our institute during 2000-2011 (including up-gradation period), the median follow-up is comparatively high.
Disease-free survival
Disease-free survival (DFS) is the length of time after treatment during which no disease is found. It can be reported for an individual patient or for a study population. Here, DFS is the length of time after treatment for a specific disease during which a patient survived with no signs of the disease worked before.
Follow-up
The patients were kept in the HEPA filter room for 1–1.5 months, followed by in-recovery transplant room for 1 month. They were advised to stay in a single room in Kolkata for 6 months and, afterwards, they were sent back home. The standard medication was suggested to be continued. The survival data is shown in Table 7.
Table 7
The survival data of overall survival, disease-free survival, rejection and mortality at median follow-up of 4.6 years (range 3 months to 10.5 years)

Long-term outcome: Relapse, mortality and rejection
There were three mortalities among our patients. No mortality occurred in the first 30 days. One non-Hodgkin lymphoma patient died on +150 day because of relapse and progressive disease, one patient of aplastic anemia died because of cytomegalo virus pneumonia and one patient of multiple myeloma had severe bronchopneumonia 10 months post-transplant. One patient died due to a road accident, but is not included in the data. The failure rate (relapse, mortality and graft rejection) is almost 32%.
Cost of therapy
The allogenic transplant cost is around 10/12 lakhs and autologous transplant cost about 8/10 lakhs in the majority of the hospitals in India. In the western countries, the cost is 10–20-times more than in India. As our NCRI is run by a charitable trust and a non-profit organization, we are performing transplants at a cost-to-cost basis. The allogenic transplant costs 5–6 lakhs in out institute, whereas the autologous transplant costs 3–4 lakhs only according to the management of post-transplant complications.
DISCUSSION
We have been able to set up a cost-effective transplant center for the first time in eastern India, and this publication will be the first published transplant data from eastern India. From August 2000 to June 2011 (with a gap of 3 years for up-gradation), a total of 22 patients were treated with stem cell transplant in the Bone Marrow Transplant unit of NCRI, Kolkata. Data on transplants performed at NCRI are being reported in the Indian Stem Cell Transplant Registry and CIBMTR (Centre for International Blood and Marrow Transplant Research).
Although the total number of transplants is small and the follow-up period is of a shorter duration, still, as a primitive center, our results are compatible with CMC, Vellore, where the number of patients was 218 and the OS was 72.3% and rejection was 14.6%.[1]
Our post-transplant complications are compatible with the results reported by other centers of India.[1,3]
Post-transplant infections as reported by CMC, Vellore, have been compared with our institute, and the comparative data has been depicted in the following table [Table 8].
Table 8
The comparison of the post-transplant infection data between our institute and other Indian centers
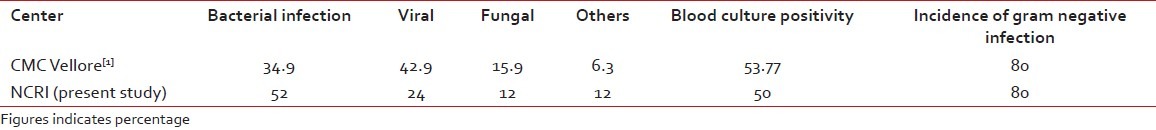
Western studies reported about 5% bacterial infection, 7% viral infection and 16% fungal infection, which is quite low in comparison with Indian reports[1,3–6] and in comparison with our present study. As the number of patients and follow-up period are less in our institute in comparison with western and Indian reports, the OS results are looking superior. A long-term follow-up with more number of patients should be carried out by our institute for real comparison in the future.
Allogenic BMT at 5–6 lakhs is equivalent to 5 years of transfusion and chelation therapy, and is a good alternative in our developing world in case of thalassaemia patients. In the present study, six thalassaemia patients were risk stratified by the criteria proposed by Lucarelli: Class I – two patients (33.33%), Class II – three patients (50.0%), Class III – one patient (16.66%), and this is a reflection of poor pre-transplant care that these patients received. Of six thalassaemia patients, four received sibling cord blood, one mixed cord blood and bone marrow and one received sibling bone marrow. All six patients were engrafted in time, but there was rejection of graft in four cases (66.66%), 3–14 months post-transplant, where we had used sibling cord blood. But, there was a 100% durable engraftment where we had used bone marrow as the stem cell source.
Human cord and placental blood provides a rich source of hematopoitic stem cells. On the basis of the finding, umbilical cord blood stem cells have been used to reconstitute hematopoiesis in children with malignant and non-malignant diseases after treatment with myeloablative doses of chemotherapy. Experience in related and unrelated donor and unrelated CBT has indicated that unrelated cord blood is associated with a slower rate of hemopoietic recovery, with a clear association between cell dose and both engraftment and survival.[7–11] Also, the low incidence of severe, acute and extensive chronic GVHD is remarkable in view of the increased use of HLA disparate grafts.[7–10,12–15] But, few reports of rejection of cord blood after engraftment are also published worldwide.[16,17] In our experience with cord blood (related and unrelated), the incidence of GVHD was less, engraftment of neutrophil and platelet was delayed but rejection rate was very high in case of thalassaemia. In other cases, there was a durable and sustainable engraftment of cord blood stem cells in case of aplastic anemia and other diseases. The data of a group of investigators suggested that the choice of cord blood graft should be based primarily on CD34 cell dose and secondarily on degree of HLA disparity.[18]
However, we can conclude that in thalassaemia, allogenic transplant with bone marrow stem cell source only is highly suggested, whereas in other indications like aplastic anemia, we may recommend to continue the transplant programme with unrelated matched or partially mismatched cord blood stem cell if no matched sibling donor is available.
We hope to continue our service to the patients of eastern India and compare our data with other state of the art transplant centers in India like CMC, Vellore, TMH, Mumbai and AIIMS, New Delhi, and abroad.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 The location of NCRI, Kolkata in the map of India

| Fig. 2 Engraftment of WBC, platelet and hemoglobin in the series of patients


 PDF
PDF  Views
Views  Share
Share

