Small HER2 Positive Breast Cancer: When is Enough
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 1-3
DOI: DOI: 10.4103/ijmpo.ijmpo_12_17
Small node negative HER2 Positive tumors are defined as T1 (<2 class="i" xss="removed">node negative). The above cohort is increasing with more awareness and acceptance of mammography screening. This group in itself is a heterogeneous population such as T1a/b versus T1c, Grade I versus II/III and estrogen receptor (ER) positive versus negative tumours. The role of adjuvant therapy for these women is a long-standing dilemma for clinicians due to the lack of prospective randomized trials and a poor representation of this group in pivotal trials. Even guidelines are inconsistent on chemotherapy regimen and the duration of trastuzumab for this subset.
There is increasing evidence from several retrospective studies for an inferior outcome in these patients with recurrence rates as high as 15%-30% ter 5-10 years.[1] In a British Columbia data set [2] of N0 breast cancers, positive HER2 status was an independent predictor of breast cancer death in 10 years in a multivariable model, with odds ratio (OR) of 2.03 (P?= 0.003) and in a European Institute of Oncology [3] population of pT1abN0 breast cancers, it was an independent predictor of 5 years disease-free survival (DFS) with OR of 2.5 (95% confidence interval [CI]: 0.9-6.5; P = 0.09). The available evidence for the efficacy of trastuzumab for these patients has limitations such as subgroup analysis of large randomized trials, retrospective nature, small numbers, few events in trials, and differing end points or durations of follow up. Five large randomized Phase III multicenter studies have shown that the addition of trastuzumab to chemotherapy results in decreased recurrence and better overall survival (OS). The proportion of TIN0 tumors and their survival outcome (subgroup analyses) compared to the overall group is depicted in [Table 1].
|
Study |
Patient population |
n |
ER+ and or PgR positive (%) |
Node negative (%) |
pT 1 tum (%) |
HR for DFS (95% CI) |
HR for OS (95% CI) |
|---|---|---|---|---|---|---|---|
|
HR given for the additional benefit for adjuvant trastuzumab compared with no such treatment. HR ? Hazard ratio; CI ? Confidence interval; DFS ? Disease-free survival; OS ? Overall survival; ER ? Estrogen receptor; ACTH ? Docetaxel and trastuzumab |
|||||||
|
HERA[4] |
HER2+N + HER2+ N- >pT1b |
3387 |
45 |
32 |
40 |
Overall: 0.54 (0.43-0.67) N-tum 0.51 (0.30-0.87) |
0.76 (0.47-1.23) |
|
N+ N- and >pT1c and PgR <10> |
1010 |
72 |
11 |
44 |
0.42 (0.21-0.83) |
0.55 (0.27-1.11) |
|
|
HER2+ N + HER2+ N+ >pT1c ER + HER2+ N- >Pt1b ER- |
3969 |
52 |
6 |
39 |
0.49 (0.41-0.58) |
0.62 (0.49-0.81) |
|
|
BCIRG 006[7] |
HER2+ N+ HER2+N- and risk factors |
3222 |
54 |
29 |
40 |
Overall; 0.49 (ACTH), 0.61 (TCH) N-negative tumors: 0.32 (0.17-0.62) Pt1c tumors: 0.6 (0.4-1.0) |
0.63 (0.48-0.81) for ACTH: 0.77 (0.60-0.99) for TCH |
|
PACS-04[8] |
HER2+ N+ |
3010 |
10 |
None |
32 |
0.86 (0.61-1.22) |
1.27 (0.68-2.38) |
Small node negative HER2 Positive tumors are defined as T1(<2>node negative). The above cohort is increasing with more awareness and acceptance of mammography screening. This group in itself is a heterogeneous population such as T1a/b versus T1c, Grade I versus II/III and estrogen receptor (ER) positive versus negative tumours. The role of adjuvant therapy for these women is a long-standing dilemma for clinicians due to the lack of prospective randomized trials and a poor representation of this group in pivotal trials. Even guidelines are inconsistent on chemotherapy regimen and the duration of trastuzumab for this subset.
There is increasing evidence from several retrospective studies for an inferior outcome in these patients with recurrence rates as high as 15%-30% ter 5-10 years.[1] In a British Columbia data set [2] of N0 breast cancers, positive HER2 status was an independent predictor of breast cancer death in 10 years in a multivariable model, with odds ratio (OR) of 2.03 (P?= 0.003) and in a European Institute of Oncology [3] population of pT1abN0 breast cancers, it was an independent predictor of 5 years disease-free survival (DFS) with OR of 2.5 (95% confidence interval [CI]: 0.9-6.5; P = 0.09). The available evidence for the efficacy of trastuzumab for these patients has limitations such as subgroup analysis of large randomized trials, retrospective nature, small numbers, few events in trials, and differing end points or durations of follow up. Five large randomized Phase III multicenter studies have shown that the addition of trastuzumab to chemotherapy results in decreased recurrence and better overall survival (OS). The proportion of TIN0 tumors and their survival outcome (subgroup analyses) compared to the overall group is depicted in [Table 1].
|
Study |
Patient population |
n |
ER+ and or PgR positive (%) |
Node negative (%) |
pT 1 tum (%) |
HR for DFS (95% CI) |
HR for OS (95% CI) |
|---|---|---|---|---|---|---|---|
|
HR given for the additional benefit for adjuvant trastuzumab compared with no such treatment. HR ? Hazard ratio; CI ? Confidence interval; DFS ? Disease-free survival; OS ? Overall survival; ER ? Estrogen receptor; ACTH ? Docetaxel and trastuzumab |
|||||||
|
HERA[4] |
HER2+N + HER2+ N- >pT1b |
3387 |
45 |
32 |
40 |
Overall: 0.54 (0.43-0.67) N-tum 0.51 (0.30-0.87) |
0.76 (0.47-1.23) |
|
N+ N- and >pT1c and PgR <10> |
1010 |
72 |
11 |
44 |
0.42 (0.21-0.83) |
0.55 (0.27-1.11) |
|
|
HER2+ N + HER2+ N+ >pT1c ER + HER2+ N- >Pt1b ER- |
3969 |
52 |
6 |
39 |
0.49 (0.41-0.58) |
0.62 (0.49-0.81) |
|
|
BCIRG 006[7] |
HER2+ N+ HER2+N- and risk factors |
3222 |
54 |
29 |
40 |
Overall; 0.49 (ACTH), 0.61 (TCH) N-negative tumors: 0.32 (0.17-0.62) Pt1c tumors: 0.6 (0.4-1.0) |
0.63 (0.48-0.81) for ACTH: 0.77 (0.60-0.99) for TCH |
|
PACS-04[8] |
HER2+ N+ |
3010 |
10 |
None |
32 |
0.86 (0.61-1.22) |
1.27 (0.68-2.38) |
|
PHARE ? Protocol of Herceptin Adjuvant with Reduced Exposure |
|
9 weeks versus 12 months |
|
Sold |
|
Doc + Tras ? FEC |
|
Doc + Tras ? FEC ? Tras |
|
Short-HER |
|
EC/AC ? Doc + Tras ? Tras |
|
Doc+Tras ? FEC |
|
6 months versus 12 months |
|
Persephone-sequential |
|
Chemotherapy ? Tras |
|
Chemotherapy ? Tras |
|
Persephone-concurrent |
|
Doc + Tras ? FEC ? Tras |
|
Doc + Tras ? FEC ? Tras |
|
PHARE |
|
Chemotherapy ? Tras |
|
Chemotherapy ? Tras |
|
Hellenic |
|
FEC ? Doc + Tras ? Tras |
|
FEC ? Doc + Tras ? Tras |
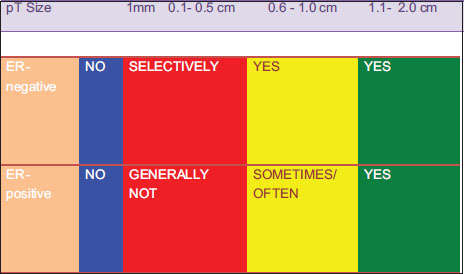
|?Figure. 1Proposed schema for adjuvant therapy of Stage 1 HER2+ breast cancer
In summary, both retrospective and meta-analysis data suggest a benefit to trastuzumab treatment in small and/or node-negative HER2+ tumors. The questions of ?how low and how much? and tumor size thresholds remain open to debate. Available data also suggests that, although durations shorter than 1 year are not clearly noninferior to 1 year of adjuvant trastuzumab, the absolute benefits of longer duration are small and may not exist in small, node negative tumors. Thus, durations shorter than 1 year may be appropriate in resource constrained settings. The potential role of novel HER2 directed therapies in this cohort is not clear. Greater integration of translation research and gene signatures may provide further insight for this subset in the future.
Conflict of Interest
There are no conflicts of interest.
References
- oerger M, Th?rlimann B, Huober J.?Small HER2-positive, node-negative breast cancer: Who should receive systemic adjuvant treatment?. Ann Oncol 2011; 22: 17-23
- hia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM.?et al.?Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 2008; 26: 5697-704
- urigliano G, Viale G, Bagnardi V, Fumagalli L, Locatelli M, Rotmensz N.?et al.?Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 2009; 27: 5693-9
- ntch M, Gelber RD, Jackisch C, Procter M, Baselga J, Bell R.?et al.?Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol 2008; 19: 1090-6
- oensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R.?et al.?Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006; 354: 809-20
- erez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CEJr.?et al?Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014; 32: 3744-52
- lamon D, Eiermann W, Robert N.?Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACT) with doxorubicin and cyclophophamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in women with Her2neu positive early breast cancer patients: BCIRG 006 study. Cancer Res 2009; 69(24 Suppl): Abstract62
- pielmann M, Roch? H, Delozier T, Canon JL, Romieu G, Bourgeois H.?et al?Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol 2009; 27: 6129-34
- olaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK.?et al.?Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015; 372: 134-41
- Jones SE, Collea R, Paul D, Sedlacek S, Favret AM, Gore IJr.?et al?Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: A single-group, open-label, phase 2 study. Lancet Oncol 2013; 14: 1121-8
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T.?et al.?6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol 2013; 14: 741-8
- Kramar A, Bachelot T, Madrange T, Pierga JY, Kerbrat P, Espi? M.?et al?Trastuzumab duration effects within patient prognostic subgroups in the PHARE trial. Ann Oncol 2014; 25: 1563-70
- Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P.?et al.?Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 2015; 26: 1333-40
Address for correspondence
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure. 1Proposed schema for adjuvant therapy of Stage 1 HER2+ breast cancer
References
- oerger M, Th?rlimann B, Huober J.?Small HER2-positive, node-negative breast cancer: Who should receive systemic adjuvant treatment?. Ann Oncol 2011; 22: 17-23
- hia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM.?et al.?Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 2008; 26: 5697-704
- urigliano G, Viale G, Bagnardi V, Fumagalli L, Locatelli M, Rotmensz N.?et al.?Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 2009; 27: 5693-9
- ntch M, Gelber RD, Jackisch C, Procter M, Baselga J, Bell R.?et al.?Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol 2008; 19: 1090-6
- oensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R.?et al.?Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006; 354: 809-20
- erez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CEJr.?et al?Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014; 32: 3744-52
- lamon D, Eiermann W, Robert N.?Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACT) with doxorubicin and cyclophophamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in women with Her2neu positive early breast cancer patients: BCIRG 006 study. Cancer Res 2009; 69(24 Suppl): Abstract62
- pielmann M, Roch? H, Delozier T, Canon JL, Romieu G, Bourgeois H.?et al?Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol 2009; 27: 6129-34
- olaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK.?et al.?Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015; 372: 134-41
- Jones SE, Collea R, Paul D, Sedlacek S, Favret AM, Gore IJr.?et al?Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: A single-group, open-label, phase 2 study. Lancet Oncol 2013; 14: 1121-8
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T.?et al.?6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol 2013; 14: 741-8
- Kramar A, Bachelot T, Madrange T, Pierga JY, Kerbrat P, Espi? M.?et al?Trastuzumab duration effects within patient prognostic subgroups in the PHARE trial. Ann Oncol 2014; 25: 1563-70
- Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P.?et al.?Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 2015; 26: 1333-40


 PDF
PDF  Views
Views  Share
Share

