Skin: A mirror of internal malignancy
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 214-222
DOI: DOI: 10.4103/0971-5851.195730
Abstract
Skin manifestations are a reflection of many of the internal diseases. Sometimes, skin disease may be the only manifestation of the internal disease. Internal malignancies may give rise to a number of cutaneous manifestations through their immunological, metabolic, and metastatic consequences. Curth proposed criteria to establish a causal relationship between a dermatosis and a malignant internal disease. Malignancy can present with a plethora of cutaneous manifestations. Here, we describe in brief about various skin manifestations of internal malignancies.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
Abstract
Skin manifestations are a reflection of many of the internal diseases. Sometimes, skin disease may be the only manifestation of the internal disease. Internal malignancies may give rise to a number of cutaneous manifestations through their immunological, metabolic, and metastatic consequences. Curth proposed criteria to establish a causal relationship between a dermatosis and a malignant internal disease. Malignancy can present with a plethora of cutaneous manifestations. Here, we describe in brief about various skin manifestations of internal malignancies.
INTRODUCTION
Skin manifestations are a reflection of many of the internal diseases; sometimes skin disease may be the only presenting complaint of many of the internal disorders. Internal malignancy, whether organ-specific or hematological can present with a plethora of cutaneous manifestations. The skin lesions can occur as secondaries or as paraneoplastic syndromes or as a part of certain genetic syndromes.[1] Internal malignancy is also one such entity which indicates its existence by various skin manifestations, so a close observation and a suspicious mind are required for detection of internal malignancy through cutaneous findings. Internal malignancies may give rise to a number of cutaneous manifestations through their immunological, metabolic, and metastatic consequences. Skin disease may sometimes provide the first clue for the diagnosis of internal malignancy. Curth proposed criteria by which a causal relationship between a dermatosis and a malignant internal disease might be evaluated.[2] These requirements include the following: (a) Both conditions start at the same time, (b) both conditions follow a parallel course, (c) the condition is not recognized as a part of a genetic syndrome, (d) a specific tumor occurs with a certain dermatosis, (e) the dermatosis is not common, and (f) a high percentage of the association is noted. In a recent Indian study, skin changes were found in 27% of patients with internal malignancies. Cutaneous metastasis was seen in 6% and other skin lesions in 25%. Secondaries in the skin usually present as papules, plaques, and nodules, common site being the anterior abdominal wall. The skin is an infrequent site for metastasis and is only the eighteenth most common site. Cutaneous metastases can arise at any age. However, in keeping with the increased incidence of malignant disease in later life, most cutaneous metastases occurred during or after the fifth decade.[3] The anterior chest wall is reported as the most common site for cutaneous metastatic lesions, and it held good for this study as well wherein 32% of the lesions were in the chest wall.[4,5] The morphological patterns of cutaneous metastases corresponded with the primary tumors and their evaluation helped localize unknown primary malignancies. In cases with known primaries, cutaneous metastases upstaged the malignancy and affected the prognosis.[6]
DIRECT TUMOR SPREAD/CUTANEOUS METASTASIS
Direct tumor spread can occasionally arise after diagnostic or therapeutic interventions such as needle aspiration of a tumor, pleural biopsy, drainage of malignant ascites, or placement of other drains in the vicinity of a tumor. “Tumor spillage,” direct contamination of wounds with tumor cells during a laparoscopy or surgical procedure, was once a problem in nearly 20% of cases but is now uncommon; laparoscopic port site metastasis rates and laparotomy wound metastasis rates due to direct tumor inoculation are both in the order of 0.8%.[7] Carcinoma erysipeloides resembles erysipelas but without the pyrexia or toxemia; tumor cells lie within dilated lymphatic vessels in the skin. This pattern is again most commonly seen in breast carcinoma but occurs due to melanoma and pelvic cancers (when it affects the lower abdomen or thigh); it can also occur due to carcinomas of the oral cavity or associated glands, as well as with upper abdominal cancers (stomach, pancreas) or lung cancer.
Cutaneous metastasis occurs in 1%–5% of all patients with internal cancers.[8] Autopsy studies suggest a higher frequency of skin metastasis than may be apparent from clinical studies; in some studies up to 9% of patients with internal cancer have had skin metastases, a large analysis suggesting that 5% is usual,[9] and in about 0.5%–1% a metastasis is the presenting feature of internal cancer.[10,11] Of patients with metastatic cancer, 10% have cutaneous metastases.[12] The most common sources of cutaneous metastases are breast, melanoma, lung, colon, stomach, upper aerodigestive tract, uterus, and kidney; the most common skin metastases from a previously unknown primary tumor originate from the kidney, lung, thyroid, or ovary.[11,13,14] Seventy-five percent of metastases are found in the 25% of body surface area that comprises the head, neck, and upper trunk.[11]
They may be the first sign of extranodal disease in up to 8% of those with metastatic cancer, especially carcinoma lung, kidney, and ovaries. Internal malignancies may metastasize to the skin by direct invasion from underlying structures, by extension through lymphatics, or by embolization into lymphatics or blood vessels. The preferential route by which tumor metastasize often determines the location of its metastasis. Carcinoma breast presents as gross lymphedema of ipsilateral limb skin ulcerations, scaly plaques of Paget's disease, or rarely Peaud'orange-like carcinoma of cuirasse. Carcinoma of oral cavity usually presents with squamous cell carcinoma (SCC) which ulcerates on the face. Lymphoma, leukemia, and myeloproliferative disorders can present with cutaneous infiltration. Renal cell carcinoma mostly metastasizes to scalp and face, but urinary tract malignancies commonly metastasize to abdominal skin. Sister Mary Joseph's nodule (an umbilicated subcutaneous nodule) arises due to metastasis from intra-abdominal malignancy chiefly gastric carcinoma. Thyroid and renal cell carcinoma present with a pulsatile tumor mass and a bruit through overlying skin. Fistula may be seen in carcinoma of urinary bladder or larynx.[15] Carcinoma en cuirasse is seen in carcinoma breast, stomach, kidney, and lungs due to carcinomatous lymphatic permeation. Carcinoma telangiectodes involve carcinoma breast. Extramammary Paget's disease is seen in in situ epithelial carcinoma, genitourinary, or gastrointestinal malignancy.
GENETICALLY DETERMINED SYNDROMES WITH RISK OF INTERNAL MALIGNANCY
A number of mechanisms underlie the association of genodermatoses with internal malignancy; these include chromosomal instability, faulty DNA repair mechanisms, abnormal lymphocyte function, and immunosurveillance, and in some cases, a combination of these. More precise genetic diagnosis, understanding of mechanisms, awareness of the benefits, and ability to focus screening of family members for genetic abnormalities or for cancers, is a constantly evolving area within dermatology and pediatric medicine in particular. Internal malignancies are linked to many of genomic instabilities. Gardner's syndrome characterized by multiple epidermoid cysts, fibromas, and pilomatrixomas. Peutz–Jeghers syndrome manifests as mucocutaneous pigmentation in peri- and intra-oral regions with risk of gastrointestinal malignancy.[16] Howel–Evans syndrome (palmoplantar keratoderma) involves the risk of esophageal carcinoma. Basal cell nevus syndrome (Gorlin's syndrome) manifests as multiple basal cell carcinomas, mandibular keratocysts, dyskeratotic pits of palms, and soles with the risk of medulloblastoma and ovarian tumors.[17] Familial melanoma syndrome (dysplastic nevus syndrome) has multiple atypical melanocytic nevi with the risk of pancreatic, gastrointestinal, lung, breast, and laryngeal carcinoma.[18] Xeroderma pigmentosum presents with severe photosensitivity, marked reduction in the threshold for sunburn, myriads of lentigines with the risk of ocular, and cutaneous malignancy [Figures [Figures11 and and22].[19] Von Hippel-Landau syndrome presents with hemangiomas and cafe-au-lait spots with risks of hemangioblastoma of the central nervous system (CNS), pheochromocytoma, renal and pancreatic adenoma, carcinoma, and cysts.[20] Neurofibromatosis type 1 and 2 presents with multiple peripheral neurofibromas, cafe-au-lait macules, axillary freckles with the risk of CNS tumors. LAMB syndrome (Carney's syndrome) is characterized by lentigines, atrial myxomas, mucocutaneous myxomas, blue nevi with the risk of gonadal hormone secreting tumors, malignant thyroid tumors [Table 1].[21] Cowden's disease (multiple hamartoma and neoplasia syndrome) shows warty “cobblestone” hyperplasia of the mucosal surfaces, particularly tongue and buccal mucosa, periorificial facial papules, acral warty keratosis, and punctuate keratosis with gastrointestinal polyposis and cysts or polyps of female genitourinary system along with breast adenocarcinoma.[22] Muir–Torre syndrome presents with sebaceous lesions and keratoacanthoma with the risk of hereditary nonpolyposis colon cancer. Ataxia telangiectasia (Louis-Bar syndrome) shows oculocutaneous telangiectasia with the risk of leukemic and breast carcinoma.[23] Bloom syndrome characterized by sun sensitive telangiectasia, cafe-au-lait macules with the risk of lymphoproliferative neoplasia. Rothmund–Thomson syndrome presents with photosensitivity, poikiloderma along with the risk of osteosarcoma and myelodysplasia.
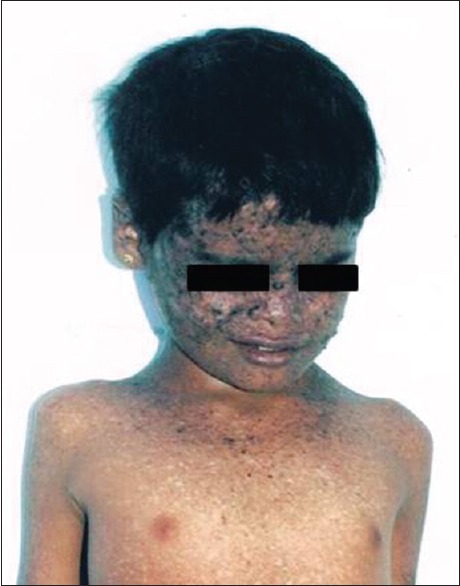
| Fig. 1 Xeroderma pigmentosum with malignant melanoma, lentigines, and hypopigmented macules in a 6-year-old girl
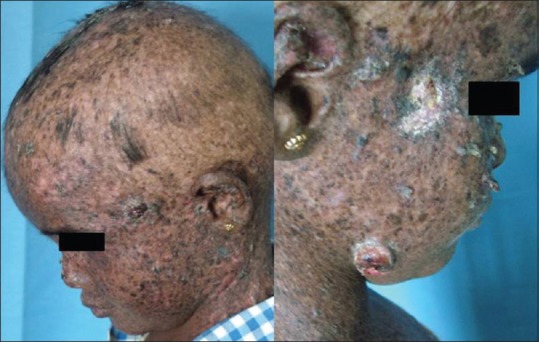
| Fig. 2 She developed squamous cell carcinoma, multiple malignant melanoma, cutaneous horn after 2 years over face, as well as trunk
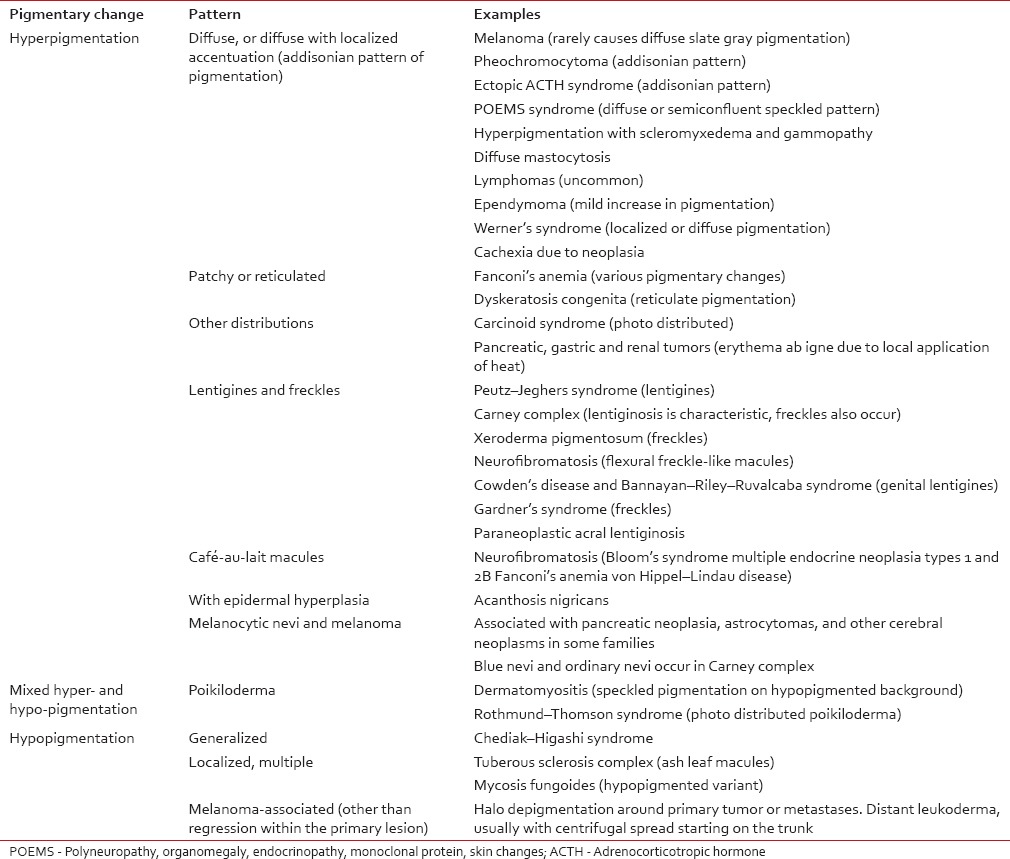
Table 1
Pigmentary abnormalities associated with internal malignancy
EXPOSURE TO CARCINOGENS
Several carcinogens such as arsenic, vinyl chloride, and radiation can produce various cutaneous manifestations. Chronic arsenic toxicity causes diffuse or spotty rain drop pigmentation, hypopigmented macules, punctuate palmoplantar keratosis, Bowen's disease, etc., vinyl chloride causes scleroderma-like skin changes with Raynaud's phenomenon and osteolysis of distal phalanges. Radiation causes radiation dermatitis of the neck which may indicate developing papillary carcinoma of the thyroid. Radiation dermatitis or multiple basal cell carcinomas over spine may indicate leukemia.
PARANEOPLASTIC SYNDROMES WITH CUTANEOUS MANIFESTATIONS
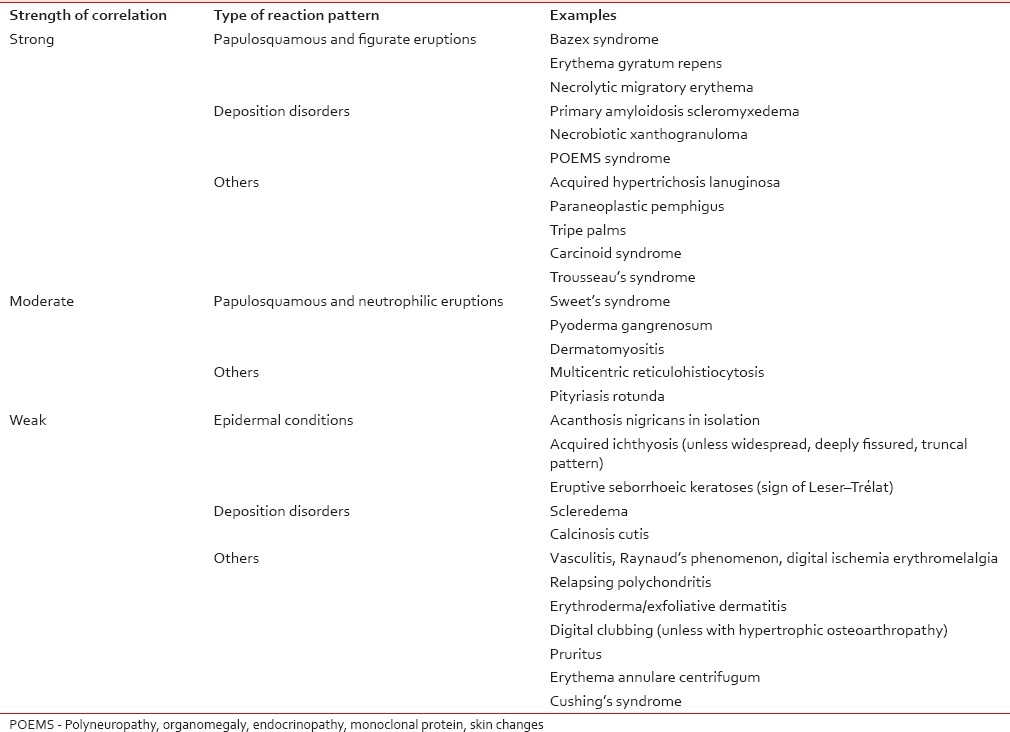
Table 2
Strength of correlation of some potentially paraneoplastic dermatoses with internal malignancy
LESIONS DUE TO DEPOSITION OF SUBSTANCES IN SKIN
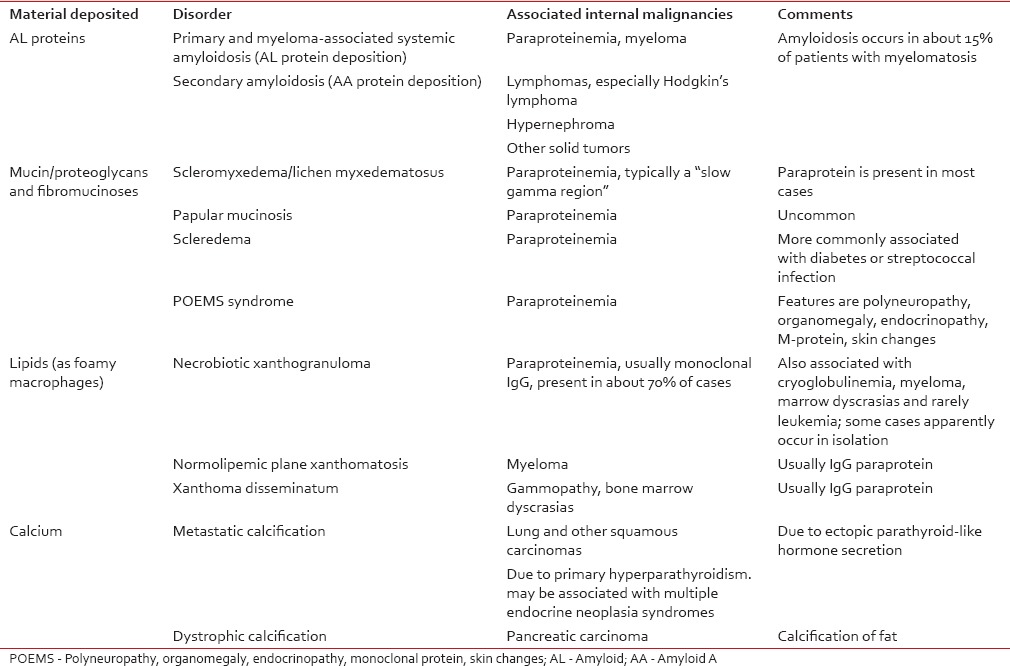
Icterus is due to extrahepatic destruction due to malignancy of gallbladder, pancreas, bile duct, or adjacent bowel. Melanosis is due to melanin which is seen in ACTH-producing tumors, primary pituitary tumor, metastasis to pituitary gland, and malignant melanoma. Hemochromatosis is diffuse gray pigmentation of skin due to hemosiderin deposition seen in hepatocellular carcinoma [Table 3].[21] Xanthomas are seen in multiple myeloma, myelocytic leukemia, myelomonocytic leukemia, diffuse histiocytic lymphoma, and cutaneous T-cell lymphoma. Systemic amyloidosis is seen in multiple myeloma.Table 3
Some deposition disorders that are linked with internal malignancy
VASCULAR AND BLOOD ABNORMALITIES
Flushing is seen in carcinoid syndrome; unilateral flushing is seen in contralateral lung cancer with Pancoast syndrome. Palmer erythema occurs in primary or metastatic liver tumor. Telangiectasias is seen in ataxia telangiectasia, Bloom's syndrome, xeroderma pigmentosum. Localized telangiectasia occurs on anterior chest wall in breast carcinoma. Generalized telangiectasia occurs in malignant angioendotheliomatosis. Progressive telangiectasia is seen in carcinoid tumors. Lymphoma is the most common cause of purpura. Acute leukemia leads to disseminated intravascular coagulation which causes purpura. Leukocytoclastic vasculitis is seen in SCC of bronchus, renal cell carcinoma, leukemia, and lymphoma.[35] Digital ischemia is seen in carcinoma pancreas, stomach, small bowel, ovary, and kidney. Migratory superficial thrombophlebitis is seen in tumors of stomach, pancreas, prostate, lung, liver, bowel, gallbladder, and ovary. Type 1 cryoglobulinemia is associated with multiple myeloma, Waldenstrom's macroglobulinemia, and B-cell malignancies. Deep vein thrombosis is associated with mucinous adenocarcinoma, myeloproliferative disorders, and metastatic cancers.[36] Erythromelalgia is seen in polycythemia vera and essential thrombocytopenia.[37]
BULLOUS DISORDERS
Paraneoplastic pemphigus seems to have an association with non-Hodgkins lymphoma, chronic lymphocytic leukemia (CLL), Castleman tumor, thymoma, etc. Pemphigus is associated with thymoma, Hodgkin's disease. Herpes gestationis is associated with hydatidiform mole and germ cell tumor. Epidermolysis bullosa acquisita is associated with carcinoma of bronchus along with amyloidosis and multiple myeloma. Linear IgA dermatosis is associated with lymphoma, CLL, carcinoma bladder and esophagus, and hydatidiform mole. Cicatricial pemphigoid is seen in carcinoma lung, stomach, colon, and endometrium.[38]
DISORDERS OF KERATINIZATION
Acanthosis nigricans is seen in gastric and intra-abdominal adenocarcinoma.[33] May be seen in carcinoma lung, uterus, esophagus, pancreas, colon, breast, ovary, or prostate, as well as lymphoma. Acquired ichthyosis is seen in Hodgkin's lymphoma, carcinoma lung, breast, and cervix. Palmer hyperkeratosis is seen in esophageal carcinoma; palmoplantar keratoderma is seen in breast or ovarian carcinoma. Tripe palms occur in gastric and lung carcinoma. Erythroderma is seen in leukemia, lymphoma. Paraneoplastic acrokeratosis of Bazex characterized by symmetric erythematous and violaceous scaly papules on hands, feet, knees, ears, and nose is seen in SCC of oropharynx, larynx, lung, esophagus, and thymus.[39] Florid cutaneous papillomatosis is characterized by multiple acuminate keratotic papules is seen in carcinoma stomach, breast, lungs, and ovary.[40] Sign of Leser–Trelat (rapid increase in size of multiple seborrheic keratosis) is seen in adenocarcinoma of stomach or colon. Multiple cherry angiomas are associated with solid tumors.[41] Multiple skin tags are associated with colonic polyps. Pityriasis rotunda characterized by fixed, annular, scaly, noninflamed, and hyperpigmented lesions on trunk occurs in hepatocellular carcinoma.
COLLAGEN VASCULAR DISEASE
Dermatomyositis is seen in carcinoma ovary, lung, pancreas, stomach, colorectal, and lymphoma. Lupus erythematosus occurs in lymphoma, thymoma. Scleroderma is seen in carcinoma lung, esophagus. Systemic lupus erythematosus is associated with lymphoreticular malignancies, myeloma, and paraproteinemias.
MULTISYSTEM AND HAEMOPOIETIC TUMORS THAT INVOLVE THE SKIN
Skin involvement occurring in these disorders ranges from very nonspecific like purpura, through to highly specific features such as cutaneous deposits of the malignancy as in lesions of leukemia cutis. There may be features that suggest a particular diagnosis like oral leukemic deposits, most typically seen in myelomonocytic leukemia, or other cutaneous disease associations as seen with neurofibromatosis type 1 associated with juvenile xanthogranuloma and juvenile myelomonocytic leukemia.[42,43,44] Involvement of the skin as a part of a multisystem tumor differs from metastases from solid tumors in both mechanism, as well as the fact that lesions are often widespread. Specific cutaneous infiltrations of the skin may occur with myeloproliferative disorders, more commonly with lymphoma, but can also occur with leukemia.[45]
INTERNAL MALIGNANCY PRESENTING AS PRURITIS
Internal carcinoma is an important cause of pruritus though it is rare and nonspecific. Mechanisms that may be involved include secondary metabolic effects like uremia, cholestasis, or due to iron-deficiency anemia, acquired ichthyosis or xerosis. Paul et al. in his 6-year study, in patients with generalized pruritus, found no significant increase in malignancy.[46] Itch may be a severe problem in patients with Hodgkin's disease and may indicate a poorer prognosis.[47] Other hematological disorders such as Sézary syndrome, mycosis fungoides, myelomatosis and leukemia, may also cause generalized pruritus.[48,49] In polycythemia rubra vera (PRV), the initiating factor appears to be rapid cooling of the skin as encountered after bathing. This is thought to be due to the release of pruritogens by degranulated mast cells.[50] However, patients with PRV can also develop intractable itching unrelated to bathing. Many other visceral carcinomas that can cause pruritus include breast and gastrointestinal cancers and carcinoid syndrome.[51]
Nerve damage by a tumor at any site can cause neuropathic pain or pruritus. The patterns that are most likely to present to a dermatologist are brachioradial pruritus and localized facial or nasal pruritus. Brachioradial pruritus[52] most commonly affects the lateral upper arm or dorsum of the forearm. Most of these cases are mechanical due to bony abnormalities, but a case due to a spinal tumor has been reported, with rapid resolution of symptoms after treatment.[53] Brain tumors are an uncommon cause of pruritus localized to the face.[52,54] Pruritus limited to the nostrils is particularly linked with tumors invading the floor of the fourth ventricle, but tumors elsewhere in the brain and other cerebral lesions (e.g., abscesses), as well as trigeminal neuralgia, can also produce this distribution of pruritus.
OTHER MANIFESTATIONS OF INTERNAL MALIGNANT DISEASES
Erythema gyratum repens characterized by erythematous bands moving in waves over the body occurs in carcinoma breast, lung, bladder, prostate, cervix, stomach, esophagus, and multiple myeloma.[55] Erythema annulare centrifugum is associated with CLL, malignant histiocytosis, Hodgkin's disease and carcinoma bronchus, nasopharynx, prostate, ovary, or rectum. Subcutaneous fat necrosis is seen in acinar cell carcinoma of the pancreas. Sweet syndrome characterized by generalized, crimson, agminate papules to large red plaques is seen in acute myelocytic leukemia, myelodysplastic syndrome, multiple myeloma, and lymphoma. Less commonly, it is seen with embryonal carcinoma of testis, ovarian carcinoma, gastric carcinoma and adenocarcinoma of breast, prostate, and rectum. Hypertrichosis lanuginosa acquisita which is the growth of villous hairs over face and ears is caused mainly by drugs but associated with tumors of colon, rectum, bladder, lung, pancreas, gallbladder, uterus, and breast.[56] Necrolytic migratory erythema characterized by erythema, vesicles, pustules, bullae and erosions involving face, intertriginous area, and perigenital region is seen in glucagonoma syndrome. Porphyria cutanea tarda is associated with liver carcinoma.[56] Pyoderma gangrenosum is associated with acute myelogenous leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, multiple myeloma, and polycythemia rubra vera. Paraneoplastic sweating is seen in Hodgkin's lymphoma, liver metastasis due to release of pyrogens from tumor. Herpes Zoster and Herpes Simplex are associated with lymphomas and leukemias. Insect bite hypersensitivity is seen in hematological malignancies like CLL and also in Ebstein–Barr virus-associated lymphoproliferative disease. Clubbing is seen in bronchogenic carcinoma and mesotheliomas.[57] Scleromyxedema is seen in multiple myeloma, Waldenstrom's macroglobulinemia, Hodgkin's or non-Hodgkins lymphoma, or leukemias.
Lichen planus may rarely be induced by neoplasia.[58]
Urticaria especially cold urticaria and peripheral gangrene as a result of circulating cryoglobulins, though uncommon, is linked with myeloma and lymphoma.[59]
Erythroderma and exfoliative dermatitis have both been linked with malignancy.[33,49] In around 10% cases, the causative neoplasm is mycosis fungoides or its leukemic variant, Sézary syndrome. There are additionally reported cases of erythroderma with cancers of liver, lung, colon, stomach, pancreas, thyroid, prostate, and cervix.[33,49,60] Ofuji papuloerythroderma has also been associated with peripheral T-cell nonepidermotrophic cutaneous lymphoma.[61] Granuloma annulare has been reported in association with lymphomas, other hematological malignancies, and uncommonly with solid tumors.[62]
“Insect bite-like” reactions are reported in hematological malignancy, usually chronic lymphocytic leukemia.[63] Cutis verticis gyrata may occasionally occur as a paraneoplastic phenomenon.[64] Mental neuropathy (“numb chin syndrome”) may occur as a feature of metastatic disease and is considered an indicator of poor prognosis.[65] Tumors causing it include breast, thyroid, renal, lung, prostate, lymphomas, and melanoma.
CONCLUSION
Skin acts like a mirror for various underlying diseases, especially malignancies. Skin manifestation may be sometimes the only symptom of underlying disease. Many underlying malignancies present with cutaneous manifestations, few of them being specific. A keen eye is, therefore, required by a dermatologist to treat all the skin conditions keeping associated malignancies in mind.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.


 PDF
PDF  Views
Views  Share
Share

