Significant Clinical Benefit of Pemetrexed-based Chemotherapy for Advanced Diffuse Malignant Peritoneal Mesothelioma: A Case Presentation
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 73-77
DOI: DOI: 10.4103/0971-5851.203495
Abstract
Diffuse malignant peritoneal mesothelioma (DMPM) is generally an understudied disease, largely because most molecular and clinical studies of mesothelioma have been conducted in patients with the more common malignant pleural mesothelioma. We present the case of a 45-year-old male that initially presented with abdominal discomfort and ascites. Diagnostic workup revealed advanced DMPM. Bimodal treatment was stared with cytoreductive surgery and hyperthermic intraperitoneal perfusion with chemotherapy procedure, followed by pemetrexed systemic monotherapy. After the disease progression, and because of a very good previous treatment response to pemetrexed, we decided to rechallenge systemic pemetrexed, along with the introduction of cisplatin. Although the intent behind systemic treatment was atfirst solely palliative, overall survival after the initial diagnosis was 50 months. Treatment based on rechallenging pemetrexed with or without cisplatin in patients with advanced DMPM can result in a quite satisfactory disease control and symptom management.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Diffuse malignant peritoneal mesothelioma (DMPM) is generally an understudied disease, largely because most molecular and clinical studies of mesothelioma have been conducted in patients with the more common malignant pleural mesothelioma. We present the case of a 45-year-old male that initially presented with abdominal discomfort and ascites. Diagnostic workup revealed advanced DMPM. Bimodal treatment was stared with cytoreductive surgery and hyperthermic intraperitoneal perfusion with chemotherapy procedure, followed by pemetrexed systemic monotherapy. After the disease progression, and because of a very good previous treatment response to pemetrexed, we decided to rechallenge systemic pemetrexed, along with the introduction of cisplatin. Although the intent behind systemic treatment was at first solely palliative, overall survival after the initial diagnosis was 50 months. Treatment based on rechallenging pemetrexed with or without cisplatin in patients with advanced DMPM can result in a quite satisfactory disease control and symptom management.
Introduction
Mesothelioma is usually considered to be an aggressive and lethal neoplasm arising from the mesothelial cells lining of pleura, peritoneum, pericardium, and tunica vaginalis testis.[1] Most frequent localization of mesothelioma is in the pleura, which accounts for about 70% of all mesotheliomas.[2] Therefore, the majority of literature focuses on the pleural variety of mesothelioma while diffuse malignant peritoneal mesothelioma (DMPM) has been evaluated to a far lesser extent, mainly because it accounts for only 10%–15% of all malignant mesotheliomas.[3] To put those percentages into perspective, it should be mentioned that incidence rates for DMPM in industrialized countries vary between 0.2 and 3 cases per million.[4] Due to the rarity of this entity, most of the available clinical information about DMPM treatment is derived from retrospective single-center series, which have inherent selection bases.[5] So far, DMPM has been poorly described with case reports being very few and far between.[6,7,8,9,10,11,12,13,14,15,16,17,18] That leads us to the fact that currently, there is no broad consensus as to what the optimal treatment for advanced stage DMPM is or should be, especially not in the form of official treatment guidelines.
Case Report
In September of 2009, a 45-year-old male patient was hospitalized complaining of periodical constipation and lower abdominal pain and presenting with a clinical syndrome of debilitating ascites. Patient was otherwise healthy, without serious illnesses in medical history, and was not taking any medications. Initial workup consisted of physical examination, routine clinical laboratory tests, echocardiography, chest X-ray, and abdominal ultrasound. Laboratory tests revealed only elevated C-reactive protein levels (101, range 0–5 mg/L). Abdominal ultrasound revealed ascites, with flank bulging and shifting dullness. Diagnostic paracentesis was conclusive for metastatic carcinoma with nonspecific cytological features. Tumor markers: Elevated CA-125-174 kIU/L (range 0–35 kIU/L), while carcinoembryonic antigen, CA 19-9, beta human chorionic gonadotropin, and alpha-fetoprotein were within normal range. Chest multislice computed tomography (MSCT) showed no evidence of intrathoracic involvement, whereas abdominal MSCT revealed a focal, localized lesion in the lower part of left kidney, peritoneal carcinomatosis, and omental cake form in the anterior abdominal wall, with a predominance of ascites. Other diagnostic procedures were also performed (abdominal X-ray, upper gastrointestinal tract endoscopy, and colonoscopy), but findings remained inconclusive. Explorative laparotomy was performed with probatory excision which discovered pelvic tumor mass infiltrating adjacent structures. Histopathology and immunochemistry staining was as follows: Calretinin and CK 5/6 were positive, whereas CD15 and BerEP4 were negative. Findings such as these are consistent with the diagnosis of a diffuse malignant peritoneal monomorphic epithelioid mesothelioma [Figures [Figures11–5]
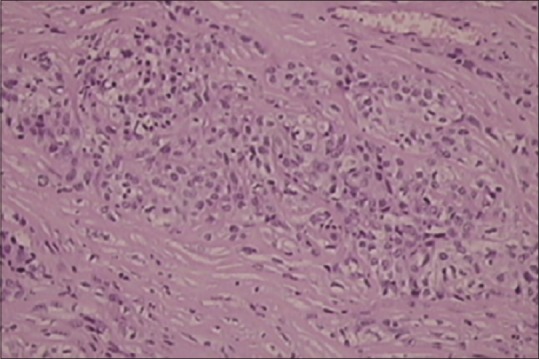
| Figure 1Histopathology showing monomorphic epithelioid peritoneal mesothelioma, (H and E, ×100)
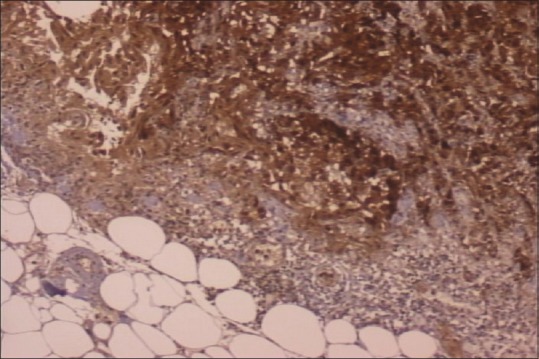
| Figure 2Calretinin-positive staining
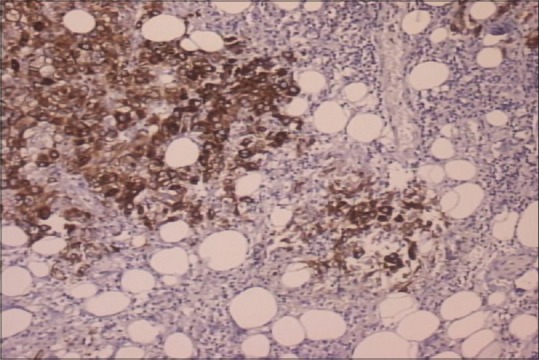
| Figure 3CK5/6 positive staining
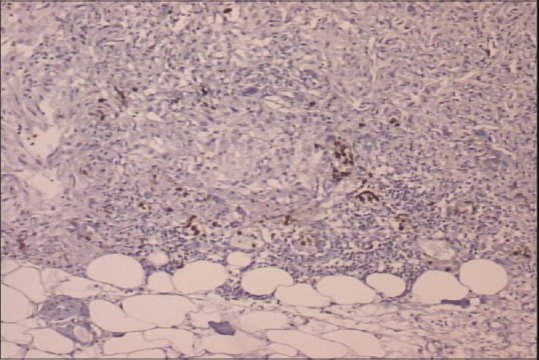
| Figure 4CD15 negative staining
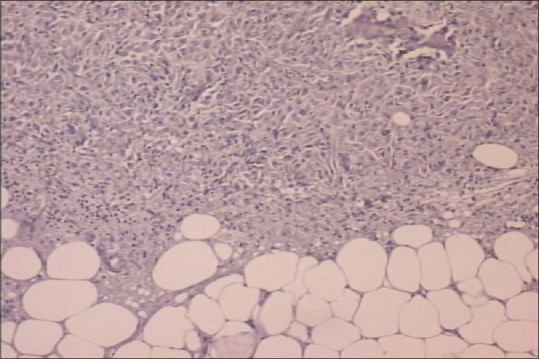
| Figure 5BerEP4 negative staining
The patient denied any previous exposure to asbestos. Incomplete cytoreductive surgery (CRS) following hyperthermic intraperitoneal perfusion with chemotherapy (HIPEC) was performed. At our institution, we use mitomycin C which was dosed at 40 mg/m2, warmed at 40°C, and then circulated true the peritoneal cavity for 90 min by perfusor.
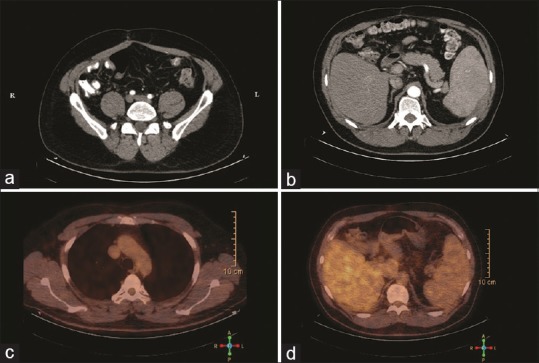
| Figure 6Radiological evaluation of diffuse malignant peritoneal mesothelioma with 18 (fludeoxyglucose) positron emission tomography-computed tomography scan performed 6 months after first-line chemotherapy. (a) Axial pelvic follow-up computed tomography demonstrates only fibrotic changes scattered throughout small bowel. (b) Axial abdominal follow-up computed tomography scan (intestine opacified with amidotrizoate) showing enlarged spleen, with smooth parietal peritoneal thickening. (c) Positron emission tomography-fused-axial chest computed tomography scan without signs of increased (18) fludeoxyglucose uptake in thoracic cavity. (d) Positron emission tomography-fused-axial abdominal computed tomography scan without detectable (18) fludeoxyglucose peritoneal uptakes
Last performed follow-up MSCT scan was 6 months after the completion of second-line chemotherapy (October 2013). Unfortunately, intra-abdominal secondary lesions further progressed. At that time, there were no available reports regarding treatment modalities for advanced DMPM, especially in the case following two lines of chemotherapy. Systemic treatment options were exhausted (due to the fact that the patient declined further gemcitabine and carboplatin treatment options that were still available), surgical treatment was not a feasible option, and radiotherapy produces questionable results due to the diffuse nature of the disease itself, while in this specific case, it was not applicable due to the location and size of DMPMs intra-abdominal deposits. Further medical approach was therefore based on best supportive care.
Discussion
Clinical and radiological presentation of early stage mesotheliomas is nonspecific. That is why most DMPMs are diagnosed in their advanced stages, with a considerable amount of time passing before reaching the correct diagnosis.[19] Pathogenesis of DMPM is still unclear, and relationship between asbestos exposure and DMPM is not nearly as straightforward as is the case with malignant pleural mesothelioma.
In a not so distant past, patients such as presented here would be considered preterminal and therefore treated only palliatively and supportively, with quite a short life expectancy. Nowadays, treatment of choice for complete cytoreduction is CRS/HIPEC. On the other hand, guidelines for systemic therapy are not that straightforward. Available scientific data support pemetrexed in combination with cisplatin as a first-line treatment option, while gemcitabine, carboplatin, and cisplatin are often considered as possible second-line treatments.[3,11,20] Due to the fragile postoperative state of the patient and because DMPM was deemed advanced at the time, single-agent pemetrexed as a palliative chemotherapy was an appropriate initial approach, even though current treatment recommendations suggest pemetrexed combined with cisplatin. Six cycles of first-line pemetrexed chemotherapy were followed by a 1-year chemotherapy-free period, as the optimal duration of chemotherapy for DMPM patients is still not established. Therefore, in this specific case of a patient with minimal burden of disease, we decided to observe for eventual disease progression. At that point, main goals were to minimize treatment toxicity and optimize patient's quality of life. After the disease progression was established, second-line chemotherapy was selected as a treatment of choice due to the satisfactory medical condition of the patient. As we already stated, data regarding the treatment of DMPM are scarce. However, Gilani et al. published a case report following their experience in the second-line treatment of advanced DMPM where they suggested that rechallenging pemetrexed with cisplatin may be a reasonable treatment option for patients who responded to the same drugs in the first line of chemotherapy.[21] In light of those facts, we sought a somewhat unconventional treatment approach, as we based second-line chemotherapy on rechallenging pemetrexed with the addition of cisplatin. Eight cycles of pemetrexed with cisplatin were applied, which lead to slower disease progression, prolonged overall survival, and better quality of life. Time to progression calculated from first- to second-line chemotherapy treatment was 12 months and overall survival after re-treatment was 7 months. Despite a highly unfavorable prognosis, patient lived for more than 50 months after the initial diagnosis. Nevertheless, it needs to be stressed out that complete recovery or even cure was never considered to be a reasonable expectation. According to the available medical reports, progression-free survival and median overall survival (OS) seem to be highly variable, with median overall survival ranging from 13.1 to 92 months.[11,22] Those periods seem to be influenced by the extent of disease at presentation, ability to surgically resect gross disease, gender, or intensity of treatment.[11,22] Physicians dealing with such a rare entity as DMPM have to bear in mind that the prognosis and overall survival of patients suffering from peritoneal mesothelioma has changed considerably during the last decade, even for the advanced stages, and that treatment of such patients should not only consist of symptomatic or palliative care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Chua TC, Yan TD, Morris DL. Surgical biology for the clinician: Peritoneal mesothelioma: Current understanding and management. Can J Surg 2009;52:59-64.
- Alexander HR. Malignant peritoneal mesothelioma: Epidemiology, risk factors, clinical presentation, diagnosis, and staging. In: Tannabe KK, editor. UpToDate, Waltham, MA. Available from: https://www.uptodate.com/contents/malignant-peritoneal-mesothelioma-epidemiology-risk-factors-clinical-presentation-diagnosis-and-staging?source=search_result&search=Alexander HR. Malignant peritoneal mesothelioma: Epidemiology, risk factors, clinical presentation, diagnosis, and staging. In: Tannabe KK, editor. UpToDate, Waltham, MA&selectedTitle=2~150. [Last accessed on 2016 Jun 13].
- Mirarabshahii P, Pillai K, Chua TC, Pourgholami MH, Morris DL. Diffuse malignant peritoneal mesothelioma – An update on treatment. Cancer Treat Rev 2012;38:605-12.
- Boffetta P. Epidemiology of peritoneal mesothelioma: A review. Ann Oncol 2007;18:985-90.
- Kindler HL. Malignant peritoneal mesothelioma. In: Tannabe KK, editor. UpToDate. Waltham, MA. Available from: https://www.uptodate.com/contents/malignant-peritoneal-mesothelioma-treatment. [Last accessed on 2016 Jun 13].
- Saraya T, Yokoyama T, Ishii H, Tanaka Y, Tsujimoto N, Ogawa Y, et al. A case of malignant peritoneal mesothelioma revealed with limitation of PET-CT in the diagnosis of thoracic metastasis. J Thorac Dis 2013;5:E11-6.
- Samedi V, White S, Zimarowski MJ, Harris A, Saffitz J, Wang HH. Metastatic peritoneal mesothelioma in the setting of recurrent ascites: A case report. Diagn Cytopathol 2010;38:675-81.
- Ito H, Imada T, Kondo J, Amano T, Maehara T, Rino Y, et al. A case of malignant peritoneal mesothelioma showed complete remission with chemotherapy. Jpn J Clin Oncol 1998;28:145-8.
- Kliem V, Maschek H, Bleck J, Schmidt FW. Malignant peritoneal mesothelioma with unusual and extensive metastasis. Dtsch Med Wochenschr 1991;116:1468-72.
- Lugaresi ML, Mattioli S, Pilotti V, Guernelli N. Pulmonary metastasis of malignant peritoneal mesothelioma. Review of the literature and a case report. Minerva Chir 2000;55:357-61.
- Nakano M, Kusaba H, Makiyama A, Ariyama H, Arita S, Oda H, et al. Pemetrexed combined with platinum-based chemotherapy for advanced malignant peritoneal mesothelioma: Retrospective analysis of six cases. Anticancer Res 2014;34:215-20.
- Tan A, Cohen P, Raoofi M, Tan J, Mesbah Ardakani N, Sterrett G. Diffuse malignant peritoneal mesothelioma presenting with psammomatous calcification on a cervical smear. Acta Cytol 2015;59:498-504.
- Pavlisko EN, Roggli VL. Sarcomatoid peritoneal mesothelioma: Clinicopathologic correlation of 13 cases. Am J Surg Pathol 2015;39:1568-75.
- Frontario SC, Loveitt A, Goldenberg-Sandau A, Liu J, Roy D, Cohen LW. Primary peritoneal mesothelioma resulting in small bowel obstruction: A case report and review of literature. Am J Case Rep 2015;16:496-500.
- Zha BS, Flanagan M, Coulson C, Garvin KW. Difficult to identify: Malignant primary peritoneal mesothelioma. Am J Med 2015;128:1191-4.
- Rupert K, Treška V. Malignant mesothelioma in the peritoneal cavity – A case report. Rozhl Chir 2015;94:166-9.
- Haberman A. Unusual appearance of malignant peritoneal mesothelioma. J Comput Assist Tomogr 2015;39:419-21.
- Kobayashi M, Moriwaki T, Ochi D, Saito R, Kobayashi K, Sugaya A, et al. A case of malignant peritoneal mesothelioma with the epithelioid histological type, successfully treated with pemetrexed plus cisplatin. Gan To Kagaku Ryoho 2014;41:361-4.
- Bridda A, Padoan I, Mencarelli R, Frego M. Peritoneal mesothelioma: A review. MedGenMed 2007;9:32.
- Kikuchi Y, Watanabe T, Igarashi Y, Sumino Y, Tsuboi K. The chemotherapy of peritoneal malignant mesothelioma. Gan To Kagaku Ryoho 2012;39:718-21.
- Gilani SN, Gridley R, Searle G, Jegannathen A. Malignant peritoneal mesothelioma (MPM) who responded to rechallenge with cisplatin and pemetrexed with current literature review. BMJ Case Rep 2013;3. Available from: http://www.casereports.bmj.com/content/2013/bcr-2012-007786.long. [Last cited on 2016 Sep 12].
- Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7.

| Figure 1Histopathology showing monomorphic epithelioid peritoneal mesothelioma, (H and E, ×100)

| Figure 2Calretinin-positive staining

| Figure 3CK5/6 positive staining

| Figure 4CD15 negative staining

| Figure 5BerEP4 negative staining

| Figure 6Radiological evaluation of diffuse malignant peritoneal mesothelioma with 18 (fludeoxyglucose) positron emission tomography-computed tomography scan performed 6 months after first-line chemotherapy. (a) Axial pelvic follow-up computed tomography demonstrates only fibrotic changes scattered throughout small bowel. (b) Axial abdominal follow-up computed tomography scan (intestine opacified with amidotrizoate) showing enlarged spleen, with smooth parietal peritoneal thickening. (c) Positron emission tomography-fused-axial chest computed tomography scan without signs of increased (18) fludeoxyglucose uptake in thoracic cavity. (d) Positron emission tomography-fused-axial abdominal computed tomography scan without detectable (18) fludeoxyglucose peritoneal uptakes
References
- Chua TC, Yan TD, Morris DL. Surgical biology for the clinician: Peritoneal mesothelioma: Current understanding and management. Can J Surg 2009;52:59-64.
- Alexander HR. Malignant peritoneal mesothelioma: Epidemiology, risk factors, clinical presentation, diagnosis, and staging. In: Tannabe KK, editor. UpToDate, Waltham, MA. Available from: https://www.uptodate.com/contents/malignant-peritoneal-mesothelioma-epidemiology-risk-factors-clinical-presentation-diagnosis-and-staging?source=search_result&search=Alexander HR. Malignant peritoneal mesothelioma: Epidemiology, risk factors, clinical presentation, diagnosis, and staging. In: Tannabe KK, editor. UpToDate, Waltham, MA&selectedTitle=2~150. [Last accessed on 2016 Jun 13].
- Mirarabshahii P, Pillai K, Chua TC, Pourgholami MH, Morris DL. Diffuse malignant peritoneal mesothelioma – An update on treatment. Cancer Treat Rev 2012;38:605-12.
- Boffetta P. Epidemiology of peritoneal mesothelioma: A review. Ann Oncol 2007;18:985-90.
- Kindler HL. Malignant peritoneal mesothelioma. In: Tannabe KK, editor. UpToDate. Waltham, MA. Available from: https://www.uptodate.com/contents/malignant-peritoneal-mesothelioma-treatment. [Last accessed on 2016 Jun 13].
- Saraya T, Yokoyama T, Ishii H, Tanaka Y, Tsujimoto N, Ogawa Y, et al. A case of malignant peritoneal mesothelioma revealed with limitation of PET-CT in the diagnosis of thoracic metastasis. J Thorac Dis 2013;5:E11-6.
- Samedi V, White S, Zimarowski MJ, Harris A, Saffitz J, Wang HH. Metastatic peritoneal mesothelioma in the setting of recurrent ascites: A case report. Diagn Cytopathol 2010;38:675-81.
- Ito H, Imada T, Kondo J, Amano T, Maehara T, Rino Y, et al. A case of malignant peritoneal mesothelioma showed complete remission with chemotherapy. Jpn J Clin Oncol 1998;28:145-8.
- Kliem V, Maschek H, Bleck J, Schmidt FW. Malignant peritoneal mesothelioma with unusual and extensive metastasis. Dtsch Med Wochenschr 1991;116:1468-72.
- Lugaresi ML, Mattioli S, Pilotti V, Guernelli N. Pulmonary metastasis of malignant peritoneal mesothelioma. Review of the literature and a case report. Minerva Chir 2000;55:357-61.
- Nakano M, Kusaba H, Makiyama A, Ariyama H, Arita S, Oda H, et al. Pemetrexed combined with platinum-based chemotherapy for advanced malignant peritoneal mesothelioma: Retrospective analysis of six cases. Anticancer Res 2014;34:215-20.
- Tan A, Cohen P, Raoofi M, Tan J, Mesbah Ardakani N, Sterrett G. Diffuse malignant peritoneal mesothelioma presenting with psammomatous calcification on a cervical smear. Acta Cytol 2015;59:498-504.
- Pavlisko EN, Roggli VL. Sarcomatoid peritoneal mesothelioma: Clinicopathologic correlation of 13 cases. Am J Surg Pathol 2015;39:1568-75.
- Frontario SC, Loveitt A, Goldenberg-Sandau A, Liu J, Roy D, Cohen LW. Primary peritoneal mesothelioma resulting in small bowel obstruction: A case report and review of literature. Am J Case Rep 2015;16:496-500.
- Zha BS, Flanagan M, Coulson C, Garvin KW. Difficult to identify: Malignant primary peritoneal mesothelioma. Am J Med 2015;128:1191-4.
- Rupert K, Treška V. Malignant mesothelioma in the peritoneal cavity – A case report. Rozhl Chir 2015;94:166-9.
- Haberman A. Unusual appearance of malignant peritoneal mesothelioma. J Comput Assist Tomogr 2015;39:419-21.
- Kobayashi M, Moriwaki T, Ochi D, Saito R, Kobayashi K, Sugaya A, et al. A case of malignant peritoneal mesothelioma with the epithelioid histological type, successfully treated with pemetrexed plus cisplatin. Gan To Kagaku Ryoho 2014;41:361-4.
- Bridda A, Padoan I, Mencarelli R, Frego M. Peritoneal mesothelioma: A review. MedGenMed 2007;9:32.
- Kikuchi Y, Watanabe T, Igarashi Y, Sumino Y, Tsuboi K. The chemotherapy of peritoneal malignant mesothelioma. Gan To Kagaku Ryoho 2012;39:718-21.
- Gilani SN, Gridley R, Searle G, Jegannathen A. Malignant peritoneal mesothelioma (MPM) who responded to rechallenge with cisplatin and pemetrexed with current literature review. BMJ Case Rep 2013;3. Available from: http://www.casereports.bmj.com/content/2013/bcr-2012-007786.long. [Last cited on 2016 Sep 12].
- Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7.


 PDF
PDF  Views
Views  Share
Share

