Sequential therapy (triple drug-based induction chemotherapy followed by concurrent chemoradiotherapy) in locally advanced inoperable head and neck cancer patients - Single institute experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(02): 86-91
DOI: DOI: 10.4103/0971-5851.89781
Abstract
Context: India has a high incidence of head and neck squamous cell carcinoma (HNSCC) mostly presenting in advanced stage. In the majority of inoperable patients a combination of chemotherapy and radiotherapy (CRT) is considered as the treatment of choice. Adding induction chemotherapy (ICT) before CRT has shown to decrease systemic relapse. Incorporation of taxanes to the cisplatin and 5-FU-based ICT has shown increase in response rates. Aims: To evaluate the efficacy and toxicity of triple drug-based ICT followed by CCRT in locally advanced, inoperable HNSCC in the Indian context. Settings and Design: Prospective, non-controlled, observational study, a single-institute experience. Materials and Methods: Consecutive, locally advanced inoperable HNSCC patients were put on sequential therapy consisting of docetaxel, 5-FU and cisplatin for three cycles followed by concurrent weekly cisplatin and radiotherapy for responding or stable disease patients. Results: Forty-four patients were enrolled with male,female ratio of 33/44(75%) and 11/44(25%). Hypopharynx 16/44(36.36%) was the most common site followed by oral cavity 12/44(27.27%) and oropharynx 12/44(27.27%); 38/44(86.36%) patients could complete the planned treatment. Seven patients required dose reduction in ICT. As per the RECIST criteria, 16 patients had Complete Response (CR) and 15 had partial response (PR), 10 had stable disease (SD) and three had progressive disease (PD) after ICT. Thirty-eight patients received concomitant chemo radiotherapy (CCRT); 28/44 (66.63%) patients achieved CR, 10/44 (22.72 %) had PR. The main toxicity was mucositis 18/44 (40.90%) secondary to ICT. Grade III and IV hematological toxicity was seen in 16/44(36.36%), of which 6/44 (13.63%) had febrile neutropenia. Conclusions: Triple drug-based sequential therapy is tolerable in our context. In this trial from a single institute the results are very encouraging.
Publication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
India has a high incidence of head and neck squamous cell carcinoma (HNSCC) mostly presenting in advanced stage. In the majority of inoperable patients a combination of chemotherapy and radiotherapy (CRT) is considered as the treatment of choice. Adding induction chemotherapy (ICT) before CRT has shown to decrease systemic relapse. Incorporation of taxanes to the cisplatin and 5-FU-based ICT has shown increase in response rates.
Aims:
To evaluate the efficacy and toxicity of triple drug-based ICT followed by CCRT in locally advanced, inoperable HNSCC in the Indian context.
Settings and Design:
Prospective, non-controlled, observational study, a single-institute experience.
Materials and Methods:
Consecutive, locally advanced inoperable HNSCC patients were put on sequential therapy consisting of docetaxel, 5-FU and cisplatin for three cycles followed by concurrent weekly cisplatin and radiotherapy for responding or stable disease patients.
Results:
Forty-four patients were enrolled with male,female ratio of 33/44(75%) and 11/44(25%). Hypopharynx 16/44(36.36%) was the most common site followed by oral cavity 12/44(27.27%) and oropharynx 12/44(27.27%); 38/44(86.36%) patients could complete the planned treatment. Seven patients required dose reduction in ICT. As per the RECIST criteria, 16 patients had Complete Response (CR) and 15 had partial response (PR), 10 had stable disease (SD) and three had progressive disease (PD) after ICT. Thirty-eight patients received concomitant chemo radiotherapy (CCRT); 28/44 (66.63%) patients achieved CR, 10/44 (22.72 %) had PR. The main toxicity was mucositis 18/44 (40.90%) secondary to ICT. Grade III and IV hematological toxicity was seen in 16/44(36.36%), of which 6/44 (13.63%) had febrile neutropenia.
Conclusions:
Triple drug-based sequential therapy is tolerable in our context. In this trial from a single institute the results are very encouraging.
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) has been considered the sixth most common cancer in the world. The incidence of HNSCC is high in developing countries like India. Use of tobacco is considered as a risk factor for the development of HNSCC. Advanced loco-regional disease, defined as either non-metastatic Stage III or Stage IV, is the most frequent clinical situation appearing in 60% of the diagnosed patients. For the loco-regional disease, an acceptable option is a local treatment based on surgery and/or radiotherapy (RT). On the other hand, in the treatment of inoperable, loco-regionally advanced HNSCC the principal treatment in most institutions is combined-modality treatment with chemoradiotherapy (CRT) if the patient is medically fit. This last approach has become the standard treatment for most patients.[1]
The Meta-Analysis of Chemotherapy on Head and Neck Cancer (MACH-NC) demonstrated that adding chemotherapy to radiotherapy in both definitive and adjuvant postoperative settings resulted in a 12% reduction in the risk of death from HNSCC, corresponding to an absolute improvement of 4% in five-year survival.[2]
Although concomitant treatment with chemotherapy and radiotherapy is the standard of treatment, several questions are still pending. For instance, retrospective studies have shown that in patients treated with CRT there was an increase in systemic relapse due to a lack of systemic control. In this regard, a renewed interest has appeared for the use of induction chemotherapy (ICT). It is considered that ICT has failed to demonstrate any survival benefit. Several meta-analyses have failed to reveal any significant improvement in survival using ICT. The largest one, as mentioned earlier, is the MACH-NC which analyzed individual patient data for more than 5200 patients. A non-significant 2% improvement in overall survival at five years was observed. However, significant survival benefits were identified in the 15 trials that employed an induction regimen using fluorouracil and platinum compounds (hazard ratio 0.88; 95% CI, 0.79-0.97).[2] On the other hand, the incorporation of taxanes into the classical cisplatin and 5-fluorouracil regimen has shown an increase in response rates.[3–5]
Docetaxel (Taxotere, Sanofi-Aventis) has substantial activity when administered alone in patients with recurrent or incurable disease.[6,7] In Phase 1 and Phase 2 studies of docetaxel with cisplatin and fluorouracil (TPF) in the treatment of locally advanced HNSCC, including Phase 2 treatment with curative intent, high clinical and pathological response rates and prolonged survival has been demonstrated.[8–12] Two Phase 3 trials in which ICT with TPF or PF was followed by radiotherapy (the European Organization for Research and Treatment of Cancer [EORTC] 24971/TAX 323 study by Vermorken et al.[13] ) or chemoradiotherapy (TAX 324) in locally advanced disease had a significantly longer survival than patients who received cisplatin and fluorouracil ICT plus chemoradiotherapy.[14]
Aims of study
The aims of study were to evaluate the efficacy and toxicity of triple drug-based ICT with docetaxel, 5-FU and cisplatin followed by concurrent chemotherapy plus radiotherapy (CRT) in patients with inoperable head and neck cancer in our context.
Primary aim of study
The primary aim of the study was overall response rate and safety analysis after triple drug-based ICT followed by CRT.
MATERIALS AND METHODS
Patients were included in the study from 1 April 2008 to 23 December 2009. Consecutive patients willing to give consent and fulfilling eligibility criteria were enrolled in the single centre at Bhagwan Mahaveer Cancer Hospital and Research Centre, Jaipur, India. Inclusion criteria were age between 18 to 75 years, histologically proven, locally advanced inoperable non-metastatic Stage IV squamous cell carcinoma of head and neck. Patients with normal liver, kidney and bone marrow functions were enrolled Pregnant and lactating females were excluded from the study. Co-morbid conditions preventing patients from completing the protocol were excluded.
Protocol
Induction chemotherapy was planned as a three-drug combination as given in the schema:
Drug Dose
Docetaxel 75 mg/m 2 Day 1
Cisplatin 75 mg/m 2 Day 1
5-FU 750 mg/m 2 Day 1,2,3 continuous infusion
- Two cycles were given initially, and in responding patients third ICT was given
- Those patients who did not show response to ICT were treated separately and were taken out of study
- ICT was followed by concurrent cisplatin as 35 mg/m 2 weekly on outpatient basis, six times during the course of radiotherapy
- Radiation therapy was given using 3D conformal radiation technique. After casting the customized thermoplastic mold, computerised tomography scan simulation was done. The images were then transferred to the Oncentra planning system. After drawing the target volumes and organs at risk on the planning scans, external beam radiation was delivered to the primary and the nodal areas using 6 MV Linear Accelerator photons. A total dose of 6600-7000 cGy/33-35 fractions @ 200cGY/ fraction five days a week over a period of six to seven weeks was delivered.
Criteria for dose reduction during induction chemo-therapy
A cycle could be delayed for up to two weeks to allow for a reduction in the severity of toxic events of Grade 3 or more to a severity of Grade 1 or less (with the exception of alopecia, fatigue, malaise, and nail changes). Delays beyond two weeks required discontinuation of chemotherapy. Reductions in the dose of docetaxel were planned for Grade 4 neutropenia and its complications, skin reactions, elevated bilirubin levels, and impaired liver function. Modifications in the dose of cisplatin were made for peripheral sensory and motor neurotoxicity, ototoxicity, or nephrotoxicity; patients with neurotoxicity or ototoxicity of Grade 3 or more were withdrawn from the study. Modifications in the dose of fluorouracil were made for mucositis and diarrhea; patients with toxic effects of Grade 4 were withdrawn from the study.
Use of Granulocyte-Colony Stimulating Factor was restricted as secondary prophylaxis only.
Response evaluation
Evaluation of response was performed at the end of ICT and again at the completion of CRT. Response was assessed by clinical and endoscopic examinations and by computerised tomography (CT) scan. RECIST criteria (RECIST1.0)[15] were used for documentation of response. Response was recorded as complete response (CR-disappearance of all target and non-target lesions) or partial response (PR-at least 30% reduction in the sum of target lesions taking as reference of the baseline sum and stable disease (SD-neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for Progressive Disease (PD- which was taken as reference of the smallest sum of longest diameter since the treatment started).
Post-treatment follow-up
First follow-up, post CCRT was after six to eight weeks and then every three months for the first two years and subsequently six-monthly. First follow-up had clinical examination, endoscopic evaluation and CT scan. Subsequently, follow-up included clinical and endoscopic evaluation only.
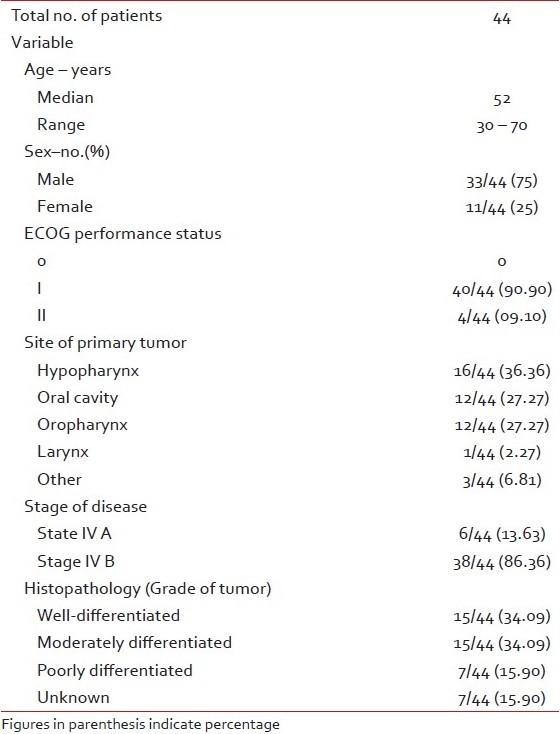
Patient characteristics
In this study total 44 patients were enrolled with the median age of 52 years (range 30–70 years). Thirty-three (75%) were male and 11(25%) were female. All the patients had Eastern Co-operative Oncology Group Performance Status I and II. The most common site was hypopharynx 16 (36.36%), followed by 12 (27.27%) patients each of the oral cavity and oropharynx. Details of the patient characteristics are enlisted in Table 1.
Table 1
Characteristics of the patients
RESULTS
Efficacy evaluation
Out of 44 patients enrolled only 38 could complete the planned study treatment. Three patients were taken out of the study because of progressive disease (PD), three patients were taken out of study because of toxicity due to ICT.
Induction therapy
Out of 44 patients six patients could not complete the planned ICT. Eighteen cycles were delayed in ICT. Ten cycles were delayed before Cycle 2 and eight cycles were delayed before the third cycle. Six patients had delay in starting CCRT. The reason for delay in treatment was hematological toxicity in most of the cases. A total six of patients required dose reduction in ICT, four patients required it at the second cycle and the remaining two required it at the start of the third cycle. Eight patients and 16/120 (13%) cycles of ICT needed G-CSF support to complete planned treatment.
Relative dose intensity of the protocol of ICT was 84%. Relative dose intensity of docetaxel was 78%.
All 44 patients were eligible for the response evaluation of ICT. Sixteen patients had complete response and 15 patients had partial response giving an overall response rate of 31/44 (70.45%). Among the partial responders nine had complete response at primary site. Ten patients had stable disease and three patients had progressive disease [Figure 1].
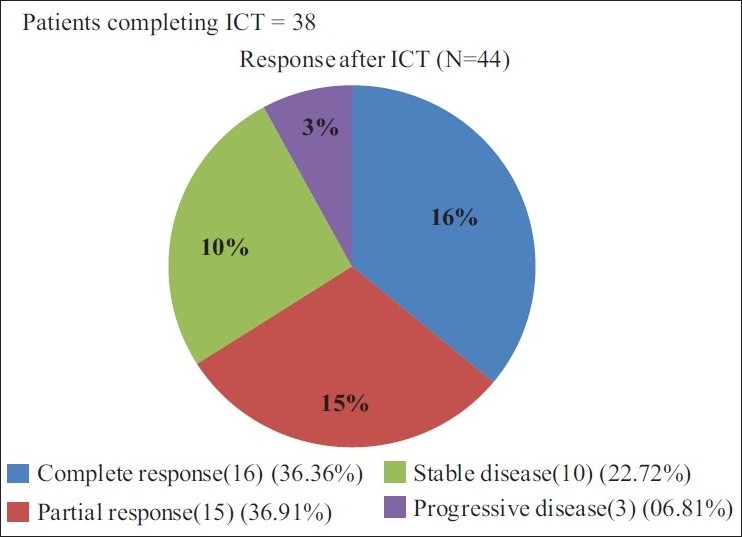
| Fig. 1 Efficacy evaluation after induction chemotherapy
Concurrent chemoradiotherapy
Out of the total 44 patients enrolled in the trial 38 patients received concurrent CRT; 28/44 (63.63%) patients achieved complete response, 10/44 (22.72%) had partial response. All the patients who could complete the treatment had a response, giving a response rate of 38/44 (86.36%) [Figure 2]. Only 4/38 (10.52%) patients required dose reduction of cisplatin during CRT. Relative dose intensity of cisplatin during CRT was 90% (31.5 mg/m 2/week).
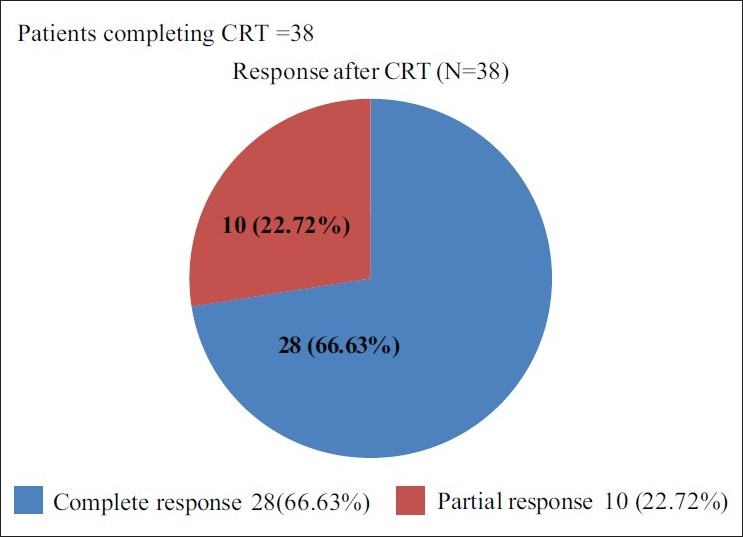
| Fig. 2 Efficacy evaluation after chemo-radiotherapy
Dose delay/ reduction in radiation was done in nine (23.68%) patients.
Post CRT: Pattern of failure
Eleven out of 38 (28.95%) patients had failure at primary site of disease, while 14/38 (36.84%) had failure at neck nodal site; 8/38 (21.05%) patients had failure at both sites.
Toxicity profile during induction chemotherapy
The main toxicity observed in this study was mucositis secondary to ICT, it was observed in 18/44 (40.90%) patients. Grade III and IV hematological toxicity was seen in 16/44(36.36%) patients, out of which six (13.63%) had febrile neutropenia, six (13.63%) had only neutropenia (Grade III or IV) and two patients each had anemia or thrombocytopenia. Eleven (25%) patients complained of vomiting and non-neutropenic infection was reported in 10 (22.72%) each. Other toxicities observed in this study were nausea, anorexia, diarrhea, lethargy etc.
Toxicity profile during induction chemotherapy
Grade III / IV mucositis was the main toxicity during CRT in 14/38(36.84%). Grade III and IV hematological toxicity in the form of neutropenia and febrile neutropenia was seen in 6/38 (15.78%) and 1/38 (02.63%) respectively [Figure 3].
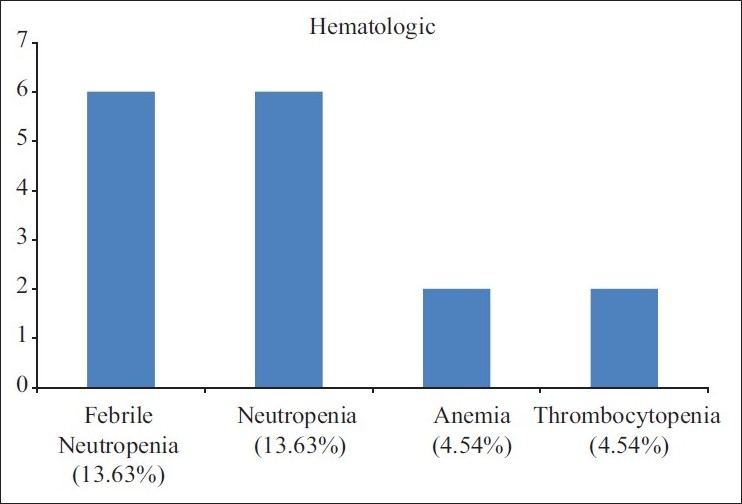
| Fig. 3 Adverse events of ICT (N=44)
DISCUSSION
Sequential therapy (ICT followed by concurrent chemotherapy plus radiotherapy) in the management of HNSCC is now an established mode of treatment though the most accepted standard of care is concurrent chemotherapy plus radiotherapy. ICT leads to better delivery of the drugs in untreated, well-vascularized tumors and helps in eradication of the micrometastatic disease with systematically active doses of chemotherapy. On the other hand, different studies have observed that CRT has improved the loco-regional control but has increased the relative risk of distant metastases (about 15-20%). The last consideration for this renewed interest in sequential treatment is the observation that a response to chemotherapy could predict a response to subsequent irradiation.
Several Phase II and Phase III trials have explored the role of three-drug combination ICT regimens with the administration of fluorouracil, cisplatin, and a taxane. In these studies, response rates higher than 90% were observed with complete responses in more than 50% of patients.[13,16] In the Phase III trial, 358 patients with unresectable disease were treated with docetaxel, cisplatin and 5-FU (DPF) or cisplatin and 5-FU (PF), followed by radiotherapy. At median follow-up of 32.5 months the DPF regimen resulted in a significantly higher Progression free survival (11.0 months vs. 8.2 months) and Over all survival (18.8 months vs. 14.5 months). This study showed the superiority of DPF in terms of not only survival, but also quality of life. Another randomized Phase III trial showed significant improvement in the response rate with the addition of a taxane in patients with locally advanced cancer of the larynx or hypopharynx. The overall response rate was significantly higher with DPF (82% vs. 60%) and more patients with DPF were able to avoid undergoing laryngectomy compared with patients receiving PF (73% vs. 63%).[17]
The role of sequential therapy by ICT with a three-drug combination of 5-FU, cisplatin and taxane followed by CRT with cisplatin and radiotherapy has also been shown to be superior to two-drug ICT. In a Phase III study conducted by Hitt et al.,[16] 383 patients received three cycles of paclitaxel and cisplatin and 5-FU (TPF) in one arm, or cisplatin and 5-FU (PF) in the other arm, followed by cisplatinum-based CRT. CR was observed in 33% in the TPF arm compared with 14% in the PF arm (P < 0.001). An increase in Time to tumor progression was observed for unresectable tumors in the TPF group (17.7 vs. 21.7). TPF patients had a trend to longer survival. Contrary to what might be expected, toxicity with paclitaxel plus cisplatin and 5-FU was less than that observed in those in the cisplatin and 5-FU arm. In another Phase III trial, TAX 324,[14] more than 500 patients with loco=regionally advanced HNSCC were treated with a sequential therapy plan of ICT with cisplatin and 5-FU with or without docetaxel followed by chemoradiation with carboplatin and surgical resection in patients with locally advanced head and neck cancer. The over all response rate after ICT trended towards an improvement with DPF regimen (72% vs. 64%). The three-year survival data, including 69% of patients who have been followed for more than three years, demonstrated a significant advantage for DPF regimen over PF (62% vs. 48%). The median overall survival was 71 months and 30 months, respectively for DPF and PF. There was better loco-regional control in the DPF arm than in the PF arm, but the incidence of distant metastases in the two arms did not differ significantly.
In our study, out of the 44 enrolled patients, all were eligible for response evaluation after ICT. Sixteen (36.36%) patients had complete response and 15 (34.09%) patients had partial response, hence the overall response observed was 31(70.45%) which is comparable with the published literature. (Overall response rate was 72% in the DPF group after ICT.)[13] 38 patients were evaluated for the response, post CRT. Out of 38 patients, 28/38 (73.68%) achieved complete response, 10/38 (26.31%) had partial response. Post sequential therapy the overall response rate observed was 38/44 (86.36%). CCRT converted an additional 10/38 (26.32%) patients into CR.
Sequential therapy was well tolerated. Out of 44 patients, three patients had to withdraw from the study due to toxicity. Eighteen cycles were delayed in ICT. A total of six patients required dose reduction in ICT. Hematological and non-hematological toxicities observed in this study were comparable with those reported in the literature for docetaxel, cisplatin and 5-FU combination.
CONCLUSION
This data coming from a single institute shows the feasibility of the use of aggressive sequential therapy in the management of locally advanced head and neck cancer in the Indian setup. It is encouraging to note that the results are comparable with published data. Long-term follow-up is planned to see the pattern of relapse and metastasis. Since head and neck cancer is a very common cancer in this part of the world and a large number of the patients present in locally advanced, inoperable stages, sequential therapy offers more aggressive treatment for such patients.
ACKNOWLEDGMENT
Manuscript assistance by, Mr. Pooran Saini – Study Assistant and Mr. Ashok Saini – Study Assistant
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.

| Fig. 1 Efficacy evaluation after induction chemotherapy

| Fig. 2 Efficacy evaluation after chemo-radiotherapy

| Fig. 3 Adverse events of ICT (N=44)


 PDF
PDF  Views
Views  Share
Share

