Second Recurrence of Aggressive Angiomyxoma of Labia Majora in a 34-Year-Old Woman: A Case Report and Review of Literature
CC BY 4.0 · Indian J Med Paediatr Oncol 2024; 45(06): 542-545
DOI: DOI: 10.1055/s-0043-1777039
Aggressive angiomyxoma (AAM) is a rare, slow-growing, benign neoplasm with high recurrence and local invasion. It is usually asymptomatic and frequently presents as a mass affecting the perineal and pelvic regions of women in reproductive age group. We present a rare case of a 34-year-old woman with second recurrence of a giant AAM arising from labia majora. The patient presented with a slow-growing pedunculated mass (around 20 × 12 cm) over the right labia majora for the past 1 year. In the last 10 years, she was operated on two different occasions (2013 and 2015) for similar lesion and was a confirmed case of AAM. Ultrasound of the lesion and magnetic resonance imaging of the abdominopelvic region was suggestive of recurrent AAM. The patient underwent en bloc dissection of the tumor with negative margin. Histopathological examination confirmed the diagnosis of recurrent AAM. En bloc dissection with negative margin leads to complete removal of tumor mass. However, long-term follow-up with annual magnetic resonance imaging is advised.
Prior Presentation of Manuscript
None.
Patient Consent
Consent to write and report this case was obtained from the patient.
Authors' Contributions
Concept: M.S., S.S.; Design: M.S., S.S.; Supervision: M.S.; Materials: M.S.; Data collection and/or processing: S.S.; Analysis and/or interpretation: S.S.; Literature search: S.S.; Writing: S.S.; Critical review: M.S., S.S.
Publication History
Article published online:
27 September 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Aggressive Angiomyxoma of the Perineum: A Rare Case Entity and Literature ReviewSumit Pandita, Asian Journal of Oncology
- Aggressive Angiomyxoma of the Perineum: A Rare Case Entity and Literature ReviewSumit Pandita, VCOT Open
- Aggressive Angiomyxoma of the Perineum: A Rare Case Entity and Literature ReviewSumit Pandita, Asian Journal of Oncology
- Individualized management of Aggressive Angiomyxoma: A case reportD Beer, Geburtshilfe und Frauenheilkunde, 2014
- Aggressive Angiomyxoma of the Vulva: A Case ReportW. Han, Geburtshilfe und Frauenheilkunde, 2008
- Life threatening rickettsiosis and the role of hemophagocytic lymphohistiocytosis syndrome (HLH): Case report of a 21-year-old womanMarine Chancel, Infectious Medicine, 2023
- Acute hemorrhagic necrotizing pancreatitis after renal transplantation: a case report and literature reviewZHANG Fei, Fudan University Journal of Medical Sciences
- HPV-negative cervical and gastric-type endocervical adenocarcinoma: one case report and literature reviewYun-zhu ZHANG, Fudan University Journal of Medical Sciences, 2023
- A HRG novel mutation associated with idiopathic portal hypertension: Case report and literature reviewShan Tang, iLIVER, 2022
- Primary nasopharyngeal carcinoma with cerebrospinal fluid EBV positivity: A case report and mini literature reviewJinli Feng, Infectious Medicine, 2024
Abstract
Aggressive angiomyxoma (AAM) is a rare, slow-growing, benign neoplasm with high recurrence and local invasion. It is usually asymptomatic and frequently presents as a mass affecting the perineal and pelvic regions of women in reproductive age group. We present a rare case of a 34-year-old woman with second recurrence of a giant AAM arising from labia majora. The patient presented with a slow-growing pedunculated mass (around 20 × 12 cm) over the right labia majora for the past 1 year. In the last 10 years, she was operated on two different occasions (2013 and 2015) for similar lesion and was a confirmed case of AAM. Ultrasound of the lesion and magnetic resonance imaging of the abdominopelvic region was suggestive of recurrent AAM. The patient underwent en bloc dissection of the tumor with negative margin. Histopathological examination confirmed the diagnosis of recurrent AAM. En bloc dissection with negative margin leads to complete removal of tumor mass. However, long-term follow-up with annual magnetic resonance imaging is advised.
Keywords
aggressive angiomyxoma - case report - labia majora - mesenchymal tumor - pelvic tumorsIntroduction
Aggressive angiomyxoma (AAM), a rare myxoid mesenchymal neoplasm, mostly affects women in reproductive age group and principally involves the perineal and pelvic regions.[1] Based on the latest World Health Organization classification of soft tissue tumors, AAM is termed as deep angiomyxoma.[2] It is benign, grows slowly and insidiously, but considered aggressive due to greater propensity for local invasion.[3] [4] Thus, to reduce this risk, tumor should be resected with wide local excision with 1 cm margin.[1] Though AAM has a moderate-to-high risk of recurrence, second recurrence is rarely reported.[5] [6] Herein, we present a case of a woman with second recurrence of giant AAM arising from labia majora.
Case Description
A 34-year-old female presented with a giant swelling over right labia majora. The swelling grew slowly over the past 1 year. The associated symptoms included perineal heaviness and abstinence due to mass. She had similar lesions in the past for which she was operated twice (2013 and 2015) at a peripheral hospital. Though surgical details were not available, histopathology report confirmed it as a case of AAM of the perineum. There was no other significant medical and family history. Local examination revealed a soft, nontender, nonhyperemic, giant pedunculated mass (≈20 × 12 cm) arising from right labia majora ([Fig. 1]). There was no evidence of discharge or detectable inguinal lymph nodes.
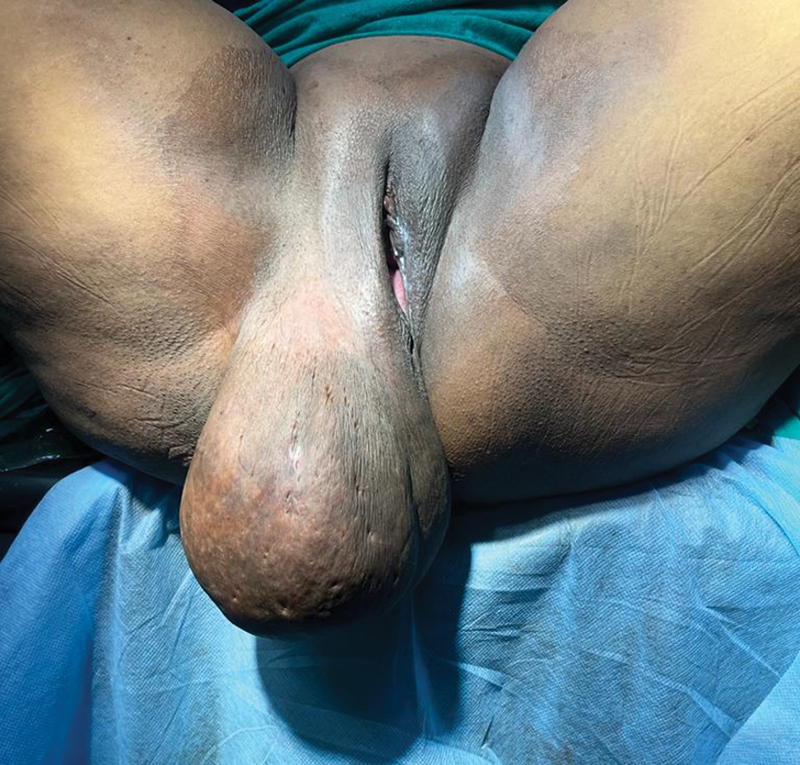
| Fig 1 Recurrent aggressive angiomyxoma arising from right labia majora.
Ultrasound examination of the perineal region demonstrated large well-defined round-to-oval heterogeneous hyperechoic lesion in the right labia majora (≈12.2 × 12.1 × 5 cm). The abdominopelvic T2-weighted magnetic resonance imaging (MRI) revealed hyperintense well-defined soft tissue lesion arising from the perineum involving the right labia majora (19 × 10 × 8.7 cm) with no signs of infiltration into adjacent structures.
The patient underwent wide local excision with negative margin and intraoperative examination revealing large pedunculated mass (25 × 17 × 12 cm) arising from the right labia majora with peduncle (5 × 5 × 5 cm). With a circumferential incision over the stalk, a sharp dissection was performed. Stalk of mass, situated in deep perineal space, was identified, clamped, and cauterized with mass en sac removed and sent for histopathological examination (HPE). The cut section was yellowish-white, with HPE revealing spindle, round, and stellate tumor cells in loose myxoid, edematous, and hypocellular stroma with variably sized blood vessels having hyalinized wall at places, suggesting recurrent AAM ([Fig. 2A] and [B]). For the last 17 months, the patient is recurrence-free following surgery.
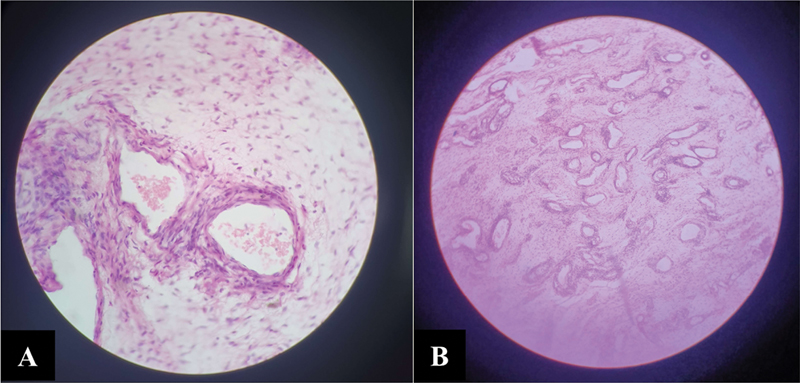
| Fig 2Histopathological examination. (A) Haphazardly dilated vessels (hematoxylin and eosin [H&E] stain, 40×). (B) Myxoid loose stroma with spindle to stellate cells (H&E stain, 4×).
Discussion
Described initially, in 1983, Steeper and Rosai[7] reported nine cases with AAM of the pelvic and perineal region. It commonly affects women (90%) of reproductive age, with highest incidence reported in those aged 20 to 50 years.[4] The predominantly involved regions include the perineum, vulva, vagina, pelvic cavity, hips, and crissum. Men are rarely affected, with a male-to-female ratio of 1:6. Though benign, AAM has a high tendency to infiltrate local tissues and recurs within 2 years of resection, at a rate of 35 to 72%.[1] [3] [4] Likewise, our case was a 34-year-old woman, with involvement of labia majora and presented with second recurrence.
Tumor cells possess estrogen (ER) and progesterone receptors (PR), and thus, tend to grow in pregnancy and are sensitive to hormonal interventions.[5] Though the pathogenesis still remains unclear, it is associated with chromosomal translocation t(8;12) induced expression of aberrant HMGIC gene and chromosomal changes in the 12q13–15 region.[3] Generally, the patient remains asymptomatic, except for a slow-growing mass. However, some of the cases may present with nonspecific symptoms, including feeling of local pressure, dull aching pain, dyspareunia, dysuria, or urinary retention.[4] On gross examination, AAM has a mean diameter of 12.7 cm (2–60 cm). It may or may not be enveloped and appear spherical or leaf-like. It is a lobulated, solid mass with soft-rubbery consistency.[1] [3] Likewise, our case presented with pedunculated, soft, lobulated, enveloped, and giant solid mass (≈20 × 12 cm).
On cut section, AAM is gelatinous and has grayish discoloration.[1] On HPE, the tumor cells appear spindle- or star-shaped, dispersed in mucinous interstitial background, with virtually absent mitoses. The tumor also shows several disordered and randomly scattered blood vessels with varying sizes and wall thickness. The blood vessels are usually surrounded by the eosinophilic spindle cells.[1] [3] Likewise, in our case, the HPE illustrated spindle, round, and stellate cells with variably sized blood vessels having hyalinized walls. Other conditions mimicking AAM on HPE include myxoma, myxofibrosarcoma, myxoid liposarcoma, and nerve sheath myxoma. However, presence of prominent vascularity separates AAM from other tumors.[4]
As AAM is a rare tumor, it is associated with 70 to 100%-chances of misdiagnosis.[4] Thus, AAM should be distinguished from other clinical conditions. Due to occurrence of AAM in the perineal and pelvic regions, it can be misdiagnosed as hernia or Bartholin cyst.[3] Moreover, owing to identical morphology, it is easy to be labeled as angiomyofibroblastoma and cellular angiofibroma, both are well circumscribed and generally do not recur.[1] [3] Thus, AAM should be diagnosed based on the clinical presentation and HPE findings.
The gross examination fails to determine the true extent of AAM. Thus, imaging studies help in reaching the diagnosis. On ultrasound, AAM appears as a cystic or hypoechoic mass.[4] Computed tomography demonstrates tumor with well-defined margin and less attenuation than muscles.[8] T2-weighted MRI illustrates tumor as a hyperintense lesion interspersed with hypointense swirled or layered strands.[1] MRI is demonstrated to be superior to computed tomography in determining the relationship of AAM to the surrounding structures.[4] Likewise, in our case, ultrasound and T2-weighted MRI enabled us to determine AAM recurrence, but MRI provided better details of tumor dimension and extent. Thus, MRI is preferred for diagnosis and follow-up.
AAM is associated with negative tumor maker (CA125, CEA, or CA199), while Hsp90 levels are raised and positively correlate with poor prognosis. Genetically, AAM shows expression of vimentin, smooth muscle actin, muscle-specific actin, desmin, CD34, F8, ER, and PR, while S-100, CK, and CD68 are absent.[1] [3] These findings highlight that AAM is characterized by differentiation into fibroblasts and muscle fibroblasts. In our case, owing to prior confirmed diagnosis of AAM, tumor markers were not evaluated. Moreover, due to poor financial status of the patient, immunohistochemistry could not be performed.
To achieve cure, and decrease the recurrence rate, three principles are described for complete resection, including the use of two incision strategy for complete tumor exposure (perineal and transabdominal incision), maintaining the intact capsule, and en bloc removal of any involved organ. Moreover, complete surgical resection with negative margin is desired. Incomplete resection is responsible for tumor seeding and recurrence.[1] Medical management with adjunct therapies, including gonadotropin-releasing hormone agonist, leads to tumor shrinkage or prevents recurrence in certain cases. Moreover, due to ER/PR positivity, targeted ER/PR therapies may be of potential value. Other modalities, including vascular embolism, may be used as adjunctive, while the role of chemotherapy and radiotherapy remains undetermined.[3] [4] [8] Likewise, in our case, single incision was used due to perineal location of the tumor and it was removed mass en sac with negative margin. Moreover, due to intact nature of the tumor, seeding at the resection site was not a concern.
Traditionally, AAM is regarded as a nonmetastasizing tumor. However, available literature suggests multiorgan metastasis in exceptional circumstances.[4] The postsurgical management of AAM is not guided by any evidence-based guidelines. However, in light of high local recurrence rate and unforeseen metastasis, patients should be followed over long term until 15 years following the primary surgical resection.
Conclusion
AAM is a benign and locally aggressive tumor. Physical examination and imaging studies help narrow down the differential diagnosis, but HPE is the confirmatory modality. En bloc surgical excision with negative margin is desired to prevent recurrence and a long-term annual follow-up with MRI is advised.
Conflict of Interest
None declared.
Acknowledgment
The authors would like to thank Dr. Vikas S. Sharma (MD), Principal Consultant, Maverick Medicorum® (India), for medical writing assistance in the preparation of this article.
Prior Presentation of Manuscript
None.
Patient Consent
Consent to write and report this case was obtained from the patient.
Authors' Contributions
Concept: M.S., S.S.; Design: M.S., S.S.; Supervision: M.S.; Materials: M.S.; Data collection and/or processing: S.S.; Analysis and/or interpretation: S.S.; Literature search: S.S.; Writing: S.S.; Critical review: M.S., S.S.
References
- Faraj W, Houjeij M, Haydar A, Nassar H, Nounou G, Khalife M. Aggressive angiomyxoma presenting with back and perineal bulge; a complex surgical approach: a case report. Int J Surg Case Rep 2016; 24: 211-214
- Wang Y, Bu X, Liu Y, Xing Y, Tong Q. Characteristics and treatment strategies of aggressive angiomyxoma in women: a retrospective review of 87 cases. Front Surg 2023; 10: 966971
- Xie Y, Qian Y, Zou B. A giant aggressive angiomyxoma of vulva in a young woman: a case report. Medicine (Baltimore) 2019; 98 (02) e13860
- Goyal LD, Garg P, Badyal R, Bhalla S. Aggressive (deep) angiomyxoma of the vulva: a case report. J Med Case Rep 2022; 16 (01) 71
- Zamani M, Mollabashi M, Mehrabi N, Alizadeh S. Aggressive angiomyxoma of vulva in 28-years old patient: a case report of second recurrence. Ann Med Surg (Lond) 2021; 69: 102706
- Aminimoghaddam S, Sarchami N, Mahboub SS. Second recurrence of aggressive vulvar angiomyxoma: a case report. J Int Med Res 2023; 51 (08) 30 00605231189366
- Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol 1983; 7: 463475
- Abduljabbar A, Wazzan M. Recurrent aggressive angiomyxoma presented with perianal mass and typical imaging swirl sign. Int J Surg Case Rep 2020; 72: 486-489
Address for correspondence
Shobhana Singh, MSDepartment of Obstetrics and Gynecology, Government Medical College and HospitalsHanuman Nagar, Medical Chowk, Ajni, Nagpur, Maharashtra 440003IndiaEmail: shobhana0312singh@gmail.comPublication History
Article published online:
27 September 2024© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- Aggressive Angiomyxoma of the Perineum: A Rare Case Entity and Literature ReviewSumit Pandita, Asian Journal of Oncology
- Aggressive Angiomyxoma of the Perineum: A Rare Case Entity and Literature ReviewSumit Pandita, VCOT Open
- Aggressive Angiomyxoma of the Perineum: A Rare Case Entity and Literature ReviewSumit Pandita, Asian Journal of Oncology
- Individualized management of Aggressive Angiomyxoma: A case reportD Beer, Geburtshilfe und Frauenheilkunde, 2014
- Aggressive Angiomyxoma of the Vulva: A Case ReportW. Han, Geburtshilfe und Frauenheilkunde, 2008
- Life threatening rickettsiosis and the role of hemophagocytic lymphohistiocytosis syndrome (HLH): Case report of a 21-year-old womanMarine Chancel, Infectious Medicine, 2023
- Acute suppurative thyroiditis: a case report and literature reviewJournal of Shanghai Jiaotong University (Medical Science), 2022
- Solid placental transmogrification of the lung: A case report and literature reviewXue-mei HA, Journal of Peking University (Health Sciences), 2023
- A HRG novel mutation associated with idiopathic portal hypertension: Case report and literature reviewShan Tang, iLIVER, 2022
- Primary nasopharyngeal carcinoma with cerebrospinal fluid EBV positivity: A case report and mini literature reviewJinli Feng, Infectious Medicine, 2024
- Aggressive Angiomyxoma of the Perineum: A Rare Case Entity and Literature Review

| Fig 1 Recurrent aggressive angiomyxoma arising from right labia majora.

| Fig 2Histopathological examination. (A) Haphazardly dilated vessels (hematoxylin and eosin [H&E] stain, 40×). (B) Myxoid loose stroma with spindle to stellate cells (H&E stain, 4×).
References
- Faraj W, Houjeij M, Haydar A, Nassar H, Nounou G, Khalife M. Aggressive angiomyxoma presenting with back and perineal bulge; a complex surgical approach: a case report. Int J Surg Case Rep 2016; 24: 211-214
- Wang Y, Bu X, Liu Y, Xing Y, Tong Q. Characteristics and treatment strategies of aggressive angiomyxoma in women: a retrospective review of 87 cases. Front Surg 2023; 10: 966971
- Xie Y, Qian Y, Zou B. A giant aggressive angiomyxoma of vulva in a young woman: a case report. Medicine (Baltimore) 2019; 98 (02) e13860
- Goyal LD, Garg P, Badyal R, Bhalla S. Aggressive (deep) angiomyxoma of the vulva: a case report. J Med Case Rep 2022; 16 (01) 71
- Zamani M, Mollabashi M, Mehrabi N, Alizadeh S. Aggressive angiomyxoma of vulva in 28-years old patient: a case report of second recurrence. Ann Med Surg (Lond) 2021; 69: 102706
- Aminimoghaddam S, Sarchami N, Mahboub SS. Second recurrence of aggressive vulvar angiomyxoma: a case report. J Int Med Res 2023; 51 (08) 30 00605231189366
- Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol 1983; 7: 463475
- Abduljabbar A, Wazzan M. Recurrent aggressive angiomyxoma presented with perianal mass and typical imaging swirl sign. Int J Surg Case Rep 2020; 72: 486-489


 PDF
PDF  Views
Views  Share
Share

