Role of Cell-Free DNA in Relapsed Head and Neck Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(01): 087-092
DOI: 10.1055/s-0044-1792008
Abstract
Introduction Owing to the aggressive biology of head and neck squamous cell carcinoma (HNSCC), new biomarkers that can facilitate the diagnosis and tracking of tumour growth are the need of the hour. Liquid biopsy has emerged as an easier tool than tissue biopsy to monitor the emergence of treatment resistance or the recurrence of disease at the molecular level.
Objectives To assess the role of cell-free DNA (cfDNA) as a biomarker for relapsed HNSCC.
Materials and Methods This study is a Phase 2 interventional study (NCT: CTRI/2020/02/023378) that assessed the response rates of a new triplet drug regimen in refractory or relapsed HNSCC. Thirty-five patients underwent blood sampling before the commencement of therapy and at 3 months of treatment. Isolation of cfDNA was done using magnetic beads (molecular weight near 170 kb) for quantification.
Results Twenty-eight patients had comparable data at baseline and after 3 months of treatment. The mean cfDNA reading at baseline was 8.9 ng/μL (range: 2.6 -7.3 ng/μL) of blood. The cfDNA concordance with clinical and radiological outcomes was 54.2%. The patients who responded to therapy were compared over time with patients who did not respond. Repeated measures testing found a significant difference (p 1?4 0.0035) in changes to the cfDNA levels of these two groups.
Conclusion This study posits the potential value of liquid biopsy in the treatment of recurrent HNSCC. Our findings prove the clinical relevance as well as limitations of cfDNA, which warrant extrapolation in an upfront setting too.
Keywords
molecular biomarker - recurrence risk - resistance - cell-free DNA - head and neck tumors - liquid biopsy - mutationsPatient Consent
Patient consent was obtained for this study.
Publication History
Article published online:
13 December 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Post-surgical SEPT9 Circulating Cell-free DNA Methylation Positivity as a Molecular Staging Parameter in Head and Neck Squamous Cell Cancer PatientsAlina Franzen, Laryngo-Rhino-Otologie, 2022
- Free Flaps in Head and Neck ReconstructionJefta Kozarski, Journal of Reconstructive Microsurgery, 2014
- Platelet-mediated T-cell alterations in head and neck cancerC Polasky, Laryngo-Rhino-Otologie, 2018
- BTLA DNA methylation and mRNA expression in head and neck squamous cell carcinomaTimo Vogt, Laryngo-Rhino-Otologie, 2022
- Second Free Flaps in Head and Neck ReconstructionAyman Abdel-Wahab Amin, Journal of Reconstructive Microsurgery, 1998
- Immune cell topography of head and neck cancerTara Muijlwijk, et al., Jitc, 2024
- Head and neck cancer and asbestos exposureBénédicte Clin, Occup Environ Med, 2022
- The DNA damage response network in the treatment of head and neck squamous cell carcinomaA. Psyrri, ESMO open, 2021
- Checkpoint blockade-induced CD8+ T cell differentiation in head and neck cancer respondersLiye Zhou, Jitc, 2022
- Cell-free DNA approaches for cancer early detection and interceptionJamie E Medina, Jitc, 2023
Abstract
Introduction Owing to the aggressive biology of head and neck squamous cell carcinoma (HNSCC), new biomarkers that can facilitate the diagnosis and tracking of tumour growth are the need of the hour. Liquid biopsy has emerged as an easier tool than tissue biopsy to monitor the emergence of treatment resistance or the recurrence of disease at the molecular level.
Objectives To assess the role of cell-free DNA (cfDNA) as a biomarker for relapsed HNSCC.
Materials and Methods This study is a Phase 2 interventional study (NCT: CTRI/2020/02/023378) that assessed the response rates of a new triplet drug regimen in refractory or relapsed HNSCC. Thirty-five patients underwent blood sampling before the commencement of therapy and at 3 months of treatment. Isolation of cfDNA was done using magnetic beads (molecular weight near 170 kb) for quantification.
Results Twenty-eight patients had comparable data at baseline and after 3 months of treatment. The mean cfDNA reading at baseline was 8.9 ng/μL (range: 2.6 -7.3 ng/μL) of blood. The cfDNA concordance with clinical and radiological outcomes was 54.2%. The patients who responded to therapy were compared over time with patients who did not respond. Repeated measures testing found a significant difference (p 1?4 0.0035) in changes to the cfDNA levels of these two groups.
Conclusion This study posits the potential value of liquid biopsy in the treatment of recurrent HNSCC. Our findings prove the clinical relevance as well as limitations of cfDNA, which warrant extrapolation in an upfront setting too.
Keywords
molecular biomarker - recurrence risk - resistance - cell-free DNA - head and neck tumors - liquid biopsy - mutationsIntroduction
Despite the progress brought about by the latest advances, head and neck squamous cell carcinoma (HNSCC) has a high relapse rate. The resultant demand for ongoing treatment has a huge impact on the health system.[1] [2] The assessment of head and neck cancer is done using clinical and radiological methods. The identification of new biomarkers that can aid the evaluation of HNSCC is an unmet need, particularly given that relapses are common and aggressive.
Liquid biopsy has expanded the range of biomarkers that can be used to analyze disease status.[3] [4] Circulating tumor DNA (ctDNA) (derived from cancer cells) has been identified as a subset of the total circulating cell-free DNA (cfDNA) in the bloodstream. While total cfDNA is formed under several conditions, ranging from infection to trauma, ctDNA is believed to be shed by tumor cells that are undergoing apoptosis or necrosis.[5] [6] Recently, cfDNA and ctDNA have been gaining popularity as novel blood biomarkers because their quantification, kinetic analysis, and molecular profiling have been found to have predictive and prognostic importance.[7]
The advent of liquid biopsy, a multimodal diagnostic tool, has drastically altered existing perspectives on the management of HNSCC.[8] Nevertheless, prospective studies identifying the role of liquid biopsy in HNSCC are limited. Saliva, tumor tissue, and plasma have been utilized for this purpose.[9] Blood (plasma) is a preferable option for quantifying cfDNA because it contains a higher concentration of ctDNA, is more stable, and is less prone to contamination, unlike saliva. It is simpler to collect and provides a more accurate snapshot of ctDNA than tumor tissue, which involves more invasive collection procedures. In this study, we envisaged utilizing cfDNA as a biomarker using plasma to identify responders or nonresponders and determined the existence of a correlation with survival outcomes in recurrent/metastatic HNSCC.
Materials and Methods
Study Design
Our study was a Phase II interventional study (NCT: CTRI/2020/02/023378) that evaluated the response rates of a new triplet regimen in relapsed/refractory head and neck cancer. This single-arm study was conducted at our head and neck cancer clinic at the All India Institute of Medical Sciences in New Delhi, after institutional ethical committee approval and Clinical Trials Registry—India registration were granted. Participating patients underwent palliative chemotherapy (every 28 days) as the intervention—EMF regimen: tablet erlotinib 150 mg OD, and injection methotrexate 40 mg/m2 and injection 5-fluorouracil 500 mg/m2 given intravenously on days 1 and 8 of every 28-day cycle. The primary objective was to assess the response rates of the triplet regimen (EMF). The sample size was calculated as per Simon's two-step design for a Phase II study. Assessments were done at 3-month intervals, using contrast-enhanced computed tomography via Response Evaluation Criteria in Solid Tumors 1.1 criteria. The primary outcome was to assess the objective response rate with the EMF regimen and has been described earlier in the study by Baa et al.[10] The secondary outcomes of the study were to assess the utility of cfDNA and circulating tumor cells (CTCs) as biomarkers, and correlation with survival outcomes in HNSCC was evaluated as an exploratory analysis. Our data on CTCs in relapsed/metastatic HNSCC has already been reported.[11] Herein, we elaborate the role of cfDNA as a predictive biomarker as a secondary focus of our research.
Inclusion criteria were as follows:
Histopathological diagnosis of squamous cell carcinoma of head and neck region.
Age > 18 and ≤ 70 years.
Eastern Cooperative Oncology Group performance status 0 to 2.
Recurrence of disease within 6 months after definitive or on palliative therapy.
Previous exposure to platinum agents either as chemotherapy or part of concurrent chemoradiotherapy.
Recurrence after treatment with PDL1 inhibitors.
Adequate organ function including absolute neutrophil count >1,000/mm3, platelets >100,000/mm3; normal liver function test (serum bilirubin < 2 mg/dL, aspartate transaminase <3X>50 mL/min).
Financial constraints for cetuximab, nivolumab, and pembrolizumab.
Measurable disease.
Exclusion criteria were as follows:
Uncontrolled severe comorbidities.
HIV positivity, HBsAg, or HCV-related hepatitis.
Nasopharyngeal carcinoma/sinonasal carcinoma.
Quantification of Cell-Free DNA
Venous blood samples (2–3 mL volume) were drawn under aseptic precautions in a BD vacutainer (K2 EDTA 10.8 mg) for the extraction of plasma. Serial plasma was collected before the initiation of therapy and again at 3 months. The sample collected was allowed to stand for 30 minutes, followed by centrifugation at 2,000 rpm for 10 minutes. Next, 1 mL of plasma was extracted and stored at −80°C. We used Maxwell kit and Promega Maxwell station to quantify cfDNA levels. The isolation of cfDNA was done using magnetic beads. The prefilled cartridges and absence of preprocessing steps help purify high-quality cfDNA in a simple three-step protocol. The use of a magnetic particle mover, as opposed to a liquid handler, offers advantages over other automated systems. The risk of cross-contamination was minimal because no liquid handling or splashing occurred during sample processing. The levels of cfDNA were directly measured on a nanodrop reader. The representative gel after the isolation of cfDNA using a magnetic bead yielded a molecular weight near 170 kb ([Fig. 1]).
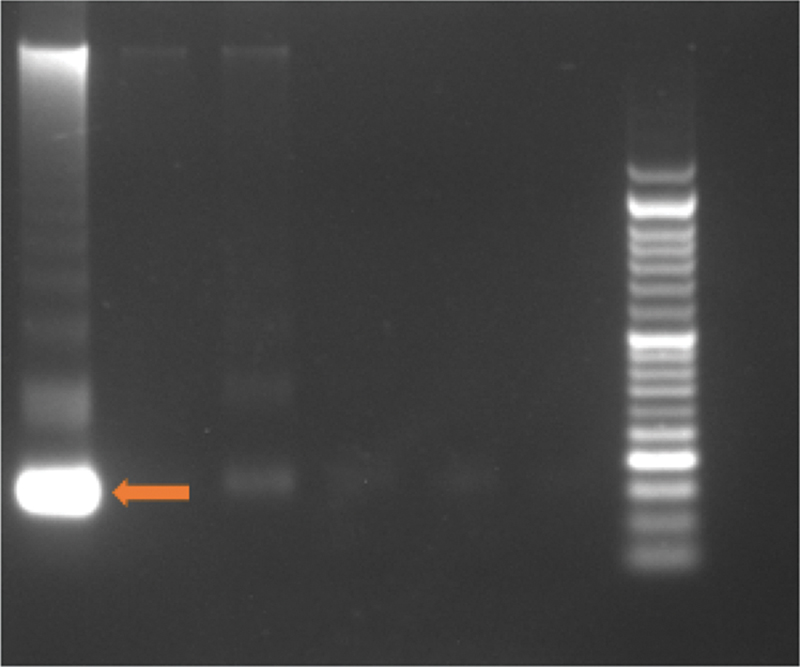
| | Fig 1Representation of gel electrophoresis of cfDNA. Arrow indicating cfDNA with a molecular weight of 170 kb. cfDNA, cell-free DNA.|
Statistical Analysis
The software package IBM SPSS v.26 was used for data analysis. Details of the baseline characteristics were reported earlier.[10] [11] The survival outcomes were assessed by Kaplan–Meier curves, and a repeated measures test was used to evaluate the difference in responses over time. The tools used in this study are similar to the tools we incorporated when we reported our overall response rates and the role of CTCs as biomarkers in relapsed/metastatic HNSCC.[10] [11]
Ethical Approval
The study was conducted after approval was granted by the Institute Ethics Committee for Postgraduate Research, All India Institute of Medical Sciences, New Delhi (ref. no. IECPG-IECPG-755/30.01.2020,OT-18/23.09.2020). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Results
Analysis of cfDNA
The mean reading at baseline was 8.9 ± 3.9 ng/µL for our entire cohort of 35 patients. Comparable data at two time points (0 and 3 months) were available only for 29 patients. The concordance and discordance rates with responses were 54.2 and 25.7, respectively, while these rates were not evaluable for 17.1%, of the patients ([Table 1]). The details of the responses (partial response/stable disease/progressive disease) achieved for each patient are presented in [Fig. 2]. A statistically significant difference (p = 0.035) in cfDNA levels with time was seen in responders and nonresponders when repeated measures time test was done. This finding corresponds with what our data described for CTCs as a marker.[11] The mean cfDNA readings declined for responders after 3 months of therapy (9.8 ± 4.5 ng/µL to 6.6 ± 3.1 ng/µL), while the nonresponders displayed an increase in their levels (7.4 ± 1.69 ng/µL to 8.5 ± 2.1 ng/µL) ([Fig. 3], [Table 2]).
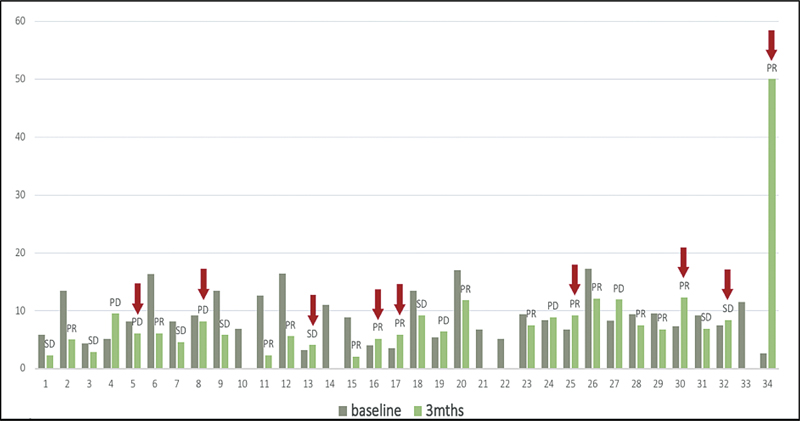
| Fig 2 Bar graph showing cfDNA levels at 0 (gray) and 3 (green) months. The response for each patient is labeled (PR/SD/PD); red arrows indicate discordance. cfDNA, cell-free DNA; PD, progressive disease; PR, partial response; SD, stable disease.|
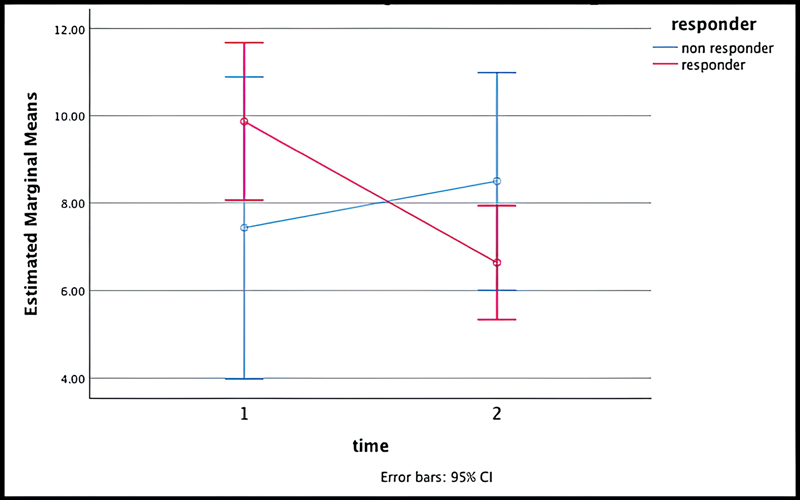
| Fig 3Repeated measures test showing difference in cfDNA levels with time in the patients who had a response (red) versus no response (blue). The difference was statistically significant (p = 0.035). cfDNA, cell-free DNA; CI, confidence interval.|
|
cfDNA at baseline |
Median PFS, mo (range) |
Median OS, mo (range) |
||
|---|---|---|---|---|
|
>9 |
6 (5.2–6.7) |
p = 0.61 |
7 (5.6–8.9) |
p = 0.98 |
|
<9> |
5 (2.9–7) |
9 (7.6–10.3) |
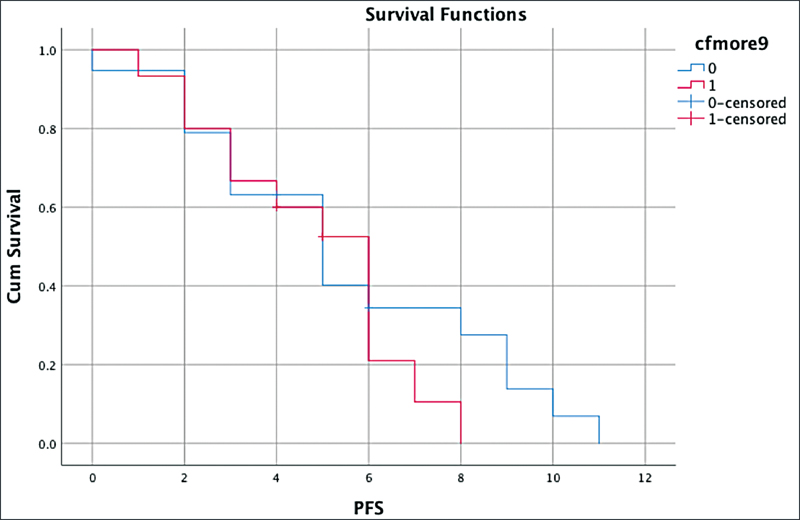
| Fig 4: Survival outcome—progression-free survival (PFS) with baseline median cell-free DNA.|
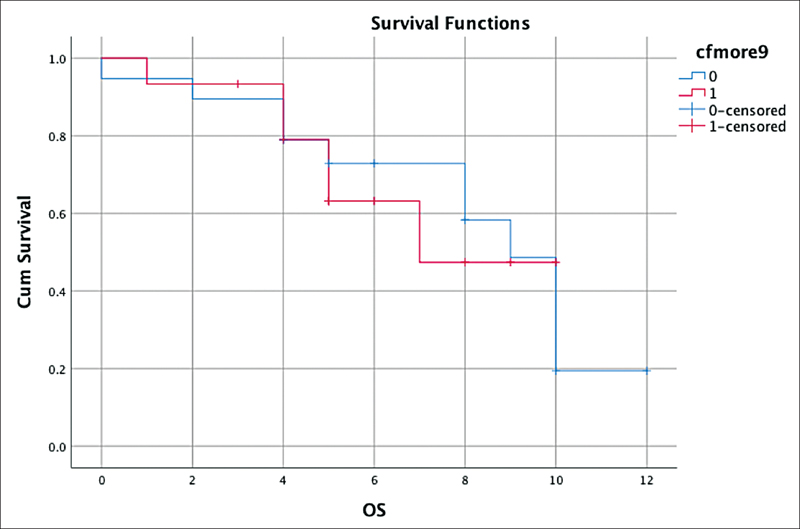
| Fig 5: Survival outcome—overall survival (OS) with baseline median cell-free DNA.|
The patients presented with a high volume of disease, characterized by swelling and ulceration (79%), followed by significant lymph node enlargement (17%). The differences in baseline cfDNA levels based on the site of origin were compared. The mean reading was higher for the tumors arising from the oral cavity (buccal mucosa predominantly) amounting to 9.39 ± 4.87 ng/µL ([Fig. 6], [Table 3]). Also, the exposure to radiotherapy as part of the concurrent chemoradiotherapy protocol was associated with lower cfDNA levels (7.59 ± 2.84 ng/µL) when compared with those who did not (11.61 ± 5.1 ng/µL) ([Table 4]).
|
Location |
Mean ± SD (ng/µL) |
|---|---|
|
Oral cavity |
9.39 ± 4.87 |
|
Oropharynx |
7.75 ± 1.89 |
|
Others (hypopharynx/larynx) |
8.53 ± 3.7 |
|
Radiation (CTRT) |
Mean ± SD (ng/µL) |
|---|---|
|
Yes |
7.59 ± 2.84 |
|
No |
11.61 ± 5.1 |
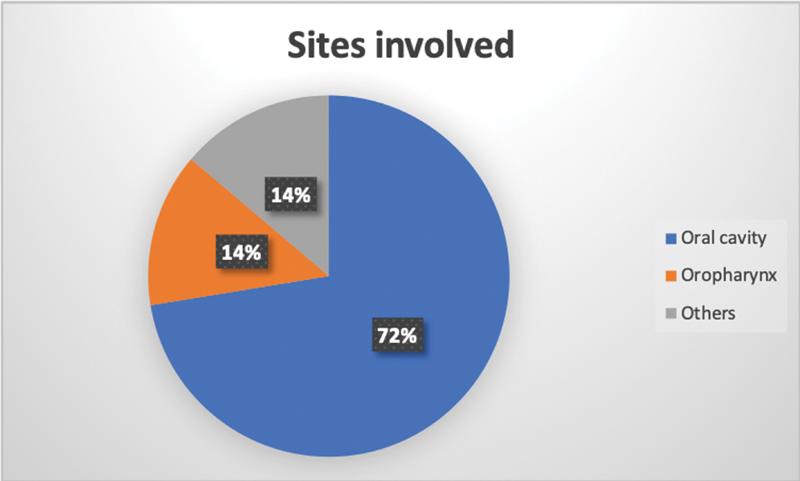
| Fig 6: Figure showing common sites involved.|
Discussion
Precision medicine has revolutionized the management of oncology. Liquid biopsy based on cfDNA analysis is one promising tool that is being explored in the treatment of many solid tumors.[3] Surani and Poterlowicz described the release of cfDNA in the system. Contributing factors are physiological mechanisms, such as apoptosis, necrosis, active secretion, and other events induced by microenvironmental stress and treatment pressure.[12] cfDNA is a combination of noncancer cell DNA from normal cells and ctDNA from cancer cells. The median cfDNA levels are higher in patients with malignancies than in healthy individuals.[3]
The mean cfDNA quantified in our cohort using the Maxwell kit (with magnetic beads) at baseline was 8.9 ± 3.9 ng/µL. Lin et al (2018) studied cfDNA levels using quantitative spectrometry in 121 patients with HNSCC. The average size reported in their study was ∼150 to 200 bp.[13] However, our study demonstrated higher cfDNA levels, probably because of the heavy burden of disease, extensive locoregional involvement, and participating patients being in a relapsed metastatic setting. Verma et al also reported high cfDNA levels in HNSCC patients undergoing chemoradiation, which correlated with a higher nodal burden.[14] This study compared the cfDNA level of patients with controls, which was lacking in our study. Verma et al used a β-globin assay to quantify the levels, while our estimation was done directly via the nanodrop technique. Another study prospectively analyzed plasma cfDNA as an early biomarker in patients undergoing radiotherapy, which showed that posttreatment higher levels were associated with early relapses.[15] However, we attempted the estimation prospectively, in a palliative setting where patients had platinum-resistant/refractory disease and the differences in cfDNA levels based on exposure to radiotherapy were revealed. The patients unexposed to radiation had a higher baseline cfDNA level compared with those receiving radiation as a part of definitive or palliative chemoradiotherapy.
Another study, by Mazurek et al, used TaqMan-based TERT amplification to quantify the cfDNA levels of 200 HNSCC patients.[16] The anatomical site (oropharyngeal vs. others) and lymph nodal burden (N2–N3 vs. N0–N1) were significantly associated with higher cfDNA levels (p = 0.011).[16] Our study supports this finding, as the mean baseline values are on the higher side. Also, the levels were more elevated in patients with tumors arising in the oral cavity, especially buccal mucosa. This concurs with the results seen in the study by Brandt et al.[9]
The concordance and discordance rates with clinical and imaging-based responses were 55.8 and 26.4%, respectively, while they could not be assessed for 14.7%, of the patients. Using the repeated measures test, a statistically significant difference (p = 0.035) in cfDNA levels was found with time between responders (decline in mean baseline cfDNA levels from 9.8 ± 4.5 to 6.6 ± 3.1 at 3 months) and nonresponders (rise in mean baseline cfDNA levels from 7.4 ± 1.69 to 8.5 ± 2.1 at 3 months) ([Fig. 3], [Table 2]).
Limitations of the Study
In this study, we have attempted to see the differences in the cfDNA levels of responders and nonresponders. This focus was lacking in previous studies. As has been reported, higher cfDNA levels are associated with a poor prognosis.[13] [14] [16] However, we did not observe any significant differences in the PFS and the OS based on a comparison of baseline cfDNA levels (> 9 or < 9). The reason may be that the crude method of cfDNA estimation leads to discordant results. The small sample size might also be a factor. Utilizing the housekeeping genes for quantification would add more robust data. Another point that must be considered is that raised cfDNA levels are also observed in infective and inflammatory conditions.[13] [14] [16] [17] [18] Therefore, the presence of comorbidities must be taken into account when interpreting the quantification of total cfDNA. Hence, the positive predictive value of cfDNA is limited by the confounding factors of poor oral hygiene, and infection that were commonly observed in our HNSCC patients.
Furthermore, as this was a single-arm study with fewer patients and no controls, there were limited data to analyze and compare. It was an exploratory analysis, as a part of our translational effort in HNSCC, and was not powered enough to establish definite conclusions and correlations. Currently, liquid biopsy is a difficult technique to access for the majority of our population. The financial constraints also pose a challenge to adequately explore this new tool.
Conclusion
This study explores cfDNA as a predictive biomarker in the treatment of HNSCC. The heavy burden on health systems of recurrent/metastatic HNSCC, with its aggressive biology and poor response rates, makes its management challenging. The identification of new biomarkers is therefore an urgent need. Our findings could be extrapolated to upfront settings to predict treatment response and detect early relapses. The incorporation of biomarkers such as cfDNA could provide invaluable support to the existing tools used in the treatment of such complicated diseases with high recurrence rates.
Conflict of Interest
None declared.
Acknowledgment
We thank our patients and family for their cooperation.
Patient Consent
Patient consent was obtained for this study.
References
- Patil VM, Noronha V, Joshi A. et al. A prospective randomized phase II study comparing metronomic chemotherapy with chemotherapy (single agent cisplatin), in patients with metastatic, relapsed or inoperable squamous cell carcinoma of head and neck. Oral Oncol 2015; 51 (03) 279-286
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- Singhal A, Hussain A, Agarwal A, Thakur B. Current status of cell-free DNA in head and neck cancer management. Annals of Indian Academy of Otorhinolaryngology Head and Neck Surgery 3 (01) 1-7
- Kong L, Birkeland AC. Liquid biopsies in head and neck cancer: current state and future challenges. Cancers (Basel) 2021; 13 (08) 1-16
- Wan JCM, Massie C, Garcia-Corbacho J. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017; 17 (04) 223-238
- Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11 (06) 426-437
- Tokuhisa Y, Iizuka N, Sakaida I. et al. Circulating cell-free DNA as a predictive marker for distant metastasis of hepatitis C virus-related hepatocellular carcinoma. Br J Cancer 2007; 97 (10) 1399-1403
- Palmirotta R, Lovero D, Cafforio P. et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol 2018; 10: 1758835918794630
- Brandt A, Thiele B, Schultheiß C, Daetwyler E, Binder M. Circulating tumor DNA in head and neck squamous cell carcinoma. Cancers (Basel) 2023; 15 (07) 15
- Baa AK, Sharma A, Bhaskar S. et al. A single-arm feasibility phase II study of EMF (erlotinib + methotrexate + 5-fluorouracil) regimen in platinum-refractory recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ecancermedicalscience 2022; 16: 1451
- Baa AK, Sharma A, Bhaskar S. et al. Role of circulating tumour cells (CTCs) in recurrent/metastatic head and neck squamous cell carcinoma (HNSCC). Ecancermedicalscience 2023; 17: 1578
- Surani A., Poterlowicz K.. (2016) “Circulating tumour DNA: a minimally invasive biomarker for tumour detection and stratification”, British Journal of Pharmacy. 1 (01) 3-18
- Lin LH, Chang KW, Kao SY, Cheng HW, Liu CJ. Increased plasma circulating cell-free DNA could be a potential marker for oral cancer. Int J Mol Sci 2018; 19 (11) 19
- Verma T, Kumari S, Mishra S. et al. Circulating free DNA as a marker of response to chemoradiation in locally advanced head and neck squamous cell carcinoma. Indian J Pathol Microbiol 2020; 63 (04) 521-526
- Koukourakis MI, Xanthopoulou E, Koukourakis IM. et al. Circulating plasma cell-free DNA (cfDNA) as a predictive biomarker for radiotherapy: results from a prospective trial in head and neck cancer. Cancer Diagn Progn 2023; 3 (05) 551-557
- Mazurek AM, Rutkowski T, Fiszer-Kierzkowska A, Małusecka E, Składowski K. Assessment of the total cfDNA and HPV16/18 detection in plasma samples of head and neck squamous cell carcinoma patients. Oral Oncol 2016; 54: 36-41
- Basnet S, Zhang ZY, Liao WQ, Li SH, Li PS, Ge HY. The prognostic value of circulating cell-free DNA in colorectal cancer: a meta-analysis. J Cancer 2016; 7 (09) 1105-1113
-
Hovhannisyan G,
Harutyunyan T,
Aroutiounian R,
Liehr T.
The diagnostic, prognostic, and therapeutic potential of cell-free DNA with a special focus on COVID-19 and other viral infections. Int J Mol Sci 2023; 24 (18) 24
Address for correspondence
Raja Pramanik, MD, DMDepartment of Medical Oncology, Dr. B.R.A. Institute Rotary Cancer Hospital, All India Institute of Medical SciencesNew Delhi 110029IndiaEmail: drrajapramanik@gmail.comPublication History
Article published online:
13 December 2024© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- Post-surgical SEPT9 Circulating Cell-free DNA Methylation Positivity as a Molecular Staging Parameter in Head and Neck Squamous Cell Cancer PatientsAlina Franzen, Laryngo-Rhino-Otologie, 2022
- Free Flaps in Head and Neck ReconstructionJefta Kozarski, Journal of Reconstructive Microsurgery, 2014
- Platelet-mediated T-cell alterations in head and neck cancerC Polasky, Laryngo-Rhino-Otologie, 2018
- BTLA DNA methylation and mRNA expression in head and neck squamous cell carcinomaTimo Vogt, Laryngo-Rhino-Otologie, 2022
- Second Free Flaps in Head and Neck ReconstructionAyman Abdel-Wahab Amin, Journal of Reconstructive Microsurgery, 1998
- Role of Taxoids in Head and Neck CancerD. Schrijvers, The Oncologist, 2000
- The role of brachytherapy in head and neck cancerC Coyle, Oxford Academic Books, 2011
- The Role Of Brachytherapy In Head And Neck CancerCatherine Coyle, Oxford Academic Books, 2005
- Head and Neck CancerCeri Hughes, Oxford Academic Books, 2008
- Head and neck cancerRuth Eakin, Oxford Academic Books, 2001
- Post-surgical SEPT9 Circulating Cell-free DNA Methylation Positivity as a Molecular Staging Parameter in Head and Neck Squamous Cell Cancer Patients

| | Fig 1Representation of gel electrophoresis of cfDNA. Arrow indicating cfDNA with a molecular weight of 170 kb. cfDNA, cell-free DNA.|

| Fig 2 Bar graph showing cfDNA levels at 0 (gray) and 3 (green) months. The response for each patient is labeled (PR/SD/PD); red arrows indicate discordance. cfDNA, cell-free DNA; PD, progressive disease; PR, partial response; SD, stable disease.|

| Fig 3Repeated measures test showing difference in cfDNA levels with time in the patients who had a response (red) versus no response (blue). The difference was statistically significant (p = 0.035). cfDNA, cell-free DNA; CI, confidence interval.|

| Fig 4: Survival outcome—progression-free survival (PFS) with baseline median cell-free DNA.|

| Fig 5: Survival outcome—overall survival (OS) with baseline median cell-free DNA.|

| Fig 6: Figure showing common sites involved.|
References
- Patil VM, Noronha V, Joshi A. et al. A prospective randomized phase II study comparing metronomic chemotherapy with chemotherapy (single agent cisplatin), in patients with metastatic, relapsed or inoperable squamous cell carcinoma of head and neck. Oral Oncol 2015; 51 (03) 279-286
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- Singhal A, Hussain A, Agarwal A, Thakur B. Current status of cell-free DNA in head and neck cancer management. Annals of Indian Academy of Otorhinolaryngology Head and Neck Surgery 3 (01) 1-7
- Kong L, Birkeland AC. Liquid biopsies in head and neck cancer: current state and future challenges. Cancers (Basel) 2021; 13 (08) 1-16
- Wan JCM, Massie C, Garcia-Corbacho J. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017; 17 (04) 223-238
- Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11 (06) 426-437
- Tokuhisa Y, Iizuka N, Sakaida I. et al. Circulating cell-free DNA as a predictive marker for distant metastasis of hepatitis C virus-related hepatocellular carcinoma. Br J Cancer 2007; 97 (10) 1399-1403
- Palmirotta R, Lovero D, Cafforio P. et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol 2018; 10: 1758835918794630
- Brandt A, Thiele B, Schultheiß C, Daetwyler E, Binder M. Circulating tumor DNA in head and neck squamous cell carcinoma. Cancers (Basel) 2023; 15 (07) 15
- Baa AK, Sharma A, Bhaskar S. et al. A single-arm feasibility phase II study of EMF (erlotinib + methotrexate + 5-fluorouracil) regimen in platinum-refractory recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ecancermedicalscience 2022; 16: 1451
- Baa AK, Sharma A, Bhaskar S. et al. Role of circulating tumour cells (CTCs) in recurrent/metastatic head and neck squamous cell carcinoma (HNSCC). Ecancermedicalscience 2023; 17: 1578
- Surani A., Poterlowicz K.. (2016) “Circulating tumour DNA: a minimally invasive biomarker for tumour detection and stratification”, British Journal of Pharmacy. 1 (01) 3-18
- Lin LH, Chang KW, Kao SY, Cheng HW, Liu CJ. Increased plasma circulating cell-free DNA could be a potential marker for oral cancer. Int J Mol Sci 2018; 19 (11) 19
- Verma T, Kumari S, Mishra S. et al. Circulating free DNA as a marker of response to chemoradiation in locally advanced head and neck squamous cell carcinoma. Indian J Pathol Microbiol 2020; 63 (04) 521-526
- Koukourakis MI, Xanthopoulou E, Koukourakis IM. et al. Circulating plasma cell-free DNA (cfDNA) as a predictive biomarker for radiotherapy: results from a prospective trial in head and neck cancer. Cancer Diagn Progn 2023; 3 (05) 551-557
- Mazurek AM, Rutkowski T, Fiszer-Kierzkowska A, Małusecka E, Składowski K. Assessment of the total cfDNA and HPV16/18 detection in plasma samples of head and neck squamous cell carcinoma patients. Oral Oncol 2016; 54: 36-41
- Basnet S, Zhang ZY, Liao WQ, Li SH, Li PS, Ge HY. The prognostic value of circulating cell-free DNA in colorectal cancer: a meta-analysis. J Cancer 2016; 7 (09) 1105-1113
- Hovhannisyan G, Harutyunyan T, Aroutiounian R, Liehr T. The diagnostic, prognostic, and therapeutic potential of cell-free DNA with a special focus on COVID-19 and other viral infections. Int J Mol Sci 2023; 24 (18) 24


 PDF
PDF  Views
Views  Share
Share

