Role of Antifungal Prophylaxis in Invasive Fungal Infection in Children with Acute Lymphoblastic Leukemia—A Retrospective Cross-Sectional Study
CC BY 4.0 · Indian J Med Paediatr Oncol 2022; 43(06): 491-499
DOI: DOI: 10.1055/s-0042-1756480
Abstract
Introduction Acute lymphoblastic leukemia (ALL) is the most common childhood cancer. Its outcome in India is not as good as that in the western world. One of the important reasons for lesser survival rates is opportunistic infections, including invasive fungal infections (IFIs). Antifungal prophylaxis (AFP) in ALL children is routinely not followed. However, owing to its incidence in high-risk ALL, this study is focused on the use of AFP in those children.
Objectives This retrospective study investigated the role of AFP in newly diagnosed children with high-risk ALL on intensive blocks of therapy on regimens B and C of the United Kingdom Acute Lymphoblastic Leukemia 2003 protocol.
Materials and Methods The study was conducted in a tertiary care center from 1st December 2013 to 31st December 2019 and included children with ALL from 1 to 18 years of age. Routine AFP with voriconazole was commenced for high-risk ALL children from 1st July 2017 onward in our center. We analyzed data of all IFIs in children before and after AFP with National Cancer Institute high-risk status who had been started on regimen B induction and regimen B or C consolidation and intensification phases.
Results A total of 55 children with high-risk ALL were included in the study. The median age was 4 years, with the majority being between the age of 1 and 10 years (38 out of 55; 65%) and predominantly male (36 out of 55; 69%). Total incidence of IFI in our cohort was 51% (28 out of 55). A significant number of children (16 out of 22 [70%]) who were not on prophylaxis developed IFI versus children (12 out of 33 [28%]) on prophylaxis (p = 0.008). The most common organisms isolated were Candida parapsilosis and Candida tropicalis. Children not receiving AFP were found to be 4.7 times (95% confidence interval: 1.44–15.13) more likely to get IFI than the ones receiving AFP. The presence of concurrent bacterial infection increases the risk of IFI (p = 0.04).
Conclusion The incidence of IFI was high in high-risk ALL children who were not on AFP. The introduction of routine AFP reduced the incidence of IFI.
Keywords
Acute lymphoblastic leukemia - fungal infection - childhood cancerDeclarations
Ethics statement
Ethical Approval (Including Committee and Record Number)
Permission granted by the Institutional Ethics committee of Kasturba Medical college hospital Mangaluru on (IEC KMC MLR 02-2021/71)
Informed Consent
Consent has been obtained initially at the time of commencement of treatment to collect the data on characteristics of acute lymphoblastic leukemia, treatment regimens, and febrile neutropenia episodes.
Author Contribution
N.S. collected and analyzed the data and drafted the manuscript, and H.P.L. conceived the idea and reviewed and edited the manuscript.
Supplementary MaterialPublication History
Article published online:
28 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Acute lymphoblastic leukemia (ALL) is the most common childhood cancer. Its outcome in India is not as good as that in the western world. One of the important reasons for lesser survival rates is opportunistic infections, including invasive fungal infections (IFIs). Antifungal prophylaxis (AFP) in ALL children is routinely not followed. However, owing to its incidence in high-risk ALL, this study is focused on the use of AFP in those children.
Objectives This retrospective study investigated the role of AFP in newly diagnosed children with high-risk ALL on intensive blocks of therapy on regimens B and C of the United Kingdom Acute Lymphoblastic Leukemia 2003 protocol.
Materials and Methods The study was conducted in a tertiary care center from 1st December 2013 to 31st December 2019 and included children with ALL from 1 to 18 years of age. Routine AFP with voriconazole was commenced for high-risk ALL children from 1st July 2017 onward in our center. We analyzed data of all IFIs in children before and after AFP with National Cancer Institute high-risk status who had been started on regimen B induction and regimen B or C consolidation and intensification phases.
Results A total of 55 children with high-risk ALL were included in the study. The median age was 4 years, with the majority being between the age of 1 and 10 years (38 out of 55; 65%) and predominantly male (36 out of 55; 69%). Total incidence of IFI in our cohort was 51% (28 out of 55). A significant number of children (16 out of 22 [70%]) who were not on prophylaxis developed IFI versus children (12 out of 33 [28%]) on prophylaxis (p = 0.008). The most common organisms isolated were Candida parapsilosis and Candida tropicalis. Children not receiving AFP were found to be 4.7 times (95% confidence interval: 1.44–15.13) more likely to get IFI than the ones receiving AFP. The presence of concurrent bacterial infection increases the risk of IFI (p = 0.04).
Conclusion The incidence of IFI was high in high-risk ALL children who were not on AFP. The introduction of routine AFP reduced the incidence of IFI.
Keywords
Acute lymphoblastic leukemia - fungal infection - childhood cancerIntroduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy worldwide, accounting for more than 25%-of all pediatric cancers.[1] Pediatric ALL is often cited as one of the true success stories of modern medicine.[2] The United Kingdom Acute Lymphoblastic Leukemia (UKALL) 2003 trial[3] [4] results have shown an overall outcome of 90%-in the United Kingdom.[3] Its event-free survival rate at 3 years in India has been observed, ranging from 41to 85%-across the country.[5] [6]
Children undergoing the treatment for cancers are at an increased risk of developing invasive fungal infections (IFIs). IFIs pose a significant challenge to the management of ALL as it results in morbidity, mortality, and interruption of treatment. Incidence of IFI was found to be high in children with acute myeloid leukemia (AML) (up to 29%), allogeneic hematopoietic stem cell transplantation (HSCT), and relapsed ALL.[7] The overall case fatality rate is about 20 to 70%, with the most inferior outcome noted with disseminated disease. Candida species was the most common organism isolated, followed by Aspergillus. Recently, Zygomycetes, Fusarium spp., and Sedoporium spp. are being increasingly observed in IFI cases.[8] [9] The fungal microflora present in our set-up, primarily consisted of Candida parapsilosis and Candida tropicalis, both of which are fluconazole resistant. Also, we wanted to target molds which remain the most common etiology for IFIs in immunocompromised patients, that is, here, ALL children. Because of these two reasons, voriconazole was chosen as antifungal prophylaxis (AFP) of choice and the antifungal policy was gradually adjusted in our medical center.
Incidence of IFI is comparatively less common during the treatment for newly diagnosed ALL than in AML/relapsed ALL/HSCT cases, and it varies depending on the protocol, regimen followed, and risk factors involved. However, there are no standard guidelines for commencing AFP in children receiving the treatment for ALL. The analysis of infection-related mortality in the UKALL 2003 protocol[10] showed that 20%-of patients had a fungal infection (predominantly Aspergillus), and it was common during the induction phase of the treatment. It is the second most common cause of infection-related mortality in ALL children. Hence, we investigated the role of AFP in children with ALL to improve the quality of life and reduce treatment-related morbidity and mortality.
Objectives
This study was to investigate the role of AFP in newly diagnosed ALL children who had National Cancer Institute (NCI) high-risk status during the intensive phases of regimens B and C, as per UKALL 2003 protocol.
Methodology
Study Population
It was a retrospective study. All children aged consecutively between the age of 1 and 18 years diagnosed with ALL who had NCI[11] high-risk status (initial white blood cells [WBC] count ≥50,000/mm3 or age ≥10 years or T cell type) between 1st December 2013 and 31st December 2019 admitted and treated at our tertiary care center, Kasturba Medical College and Hospital, Mangalore, were included. All the details of children with the type of leukemia (B or T cell), age at diagnosis, date of initiation of treatment, regimen, postinduction medical residual disease status, details about febrile neutropenia episodes, event dates (relapse and death) during the induction, consolidation, interim maintenance or escalating Capizzi, and delayed intensification blocks were obtained using structured proforma through review of medical records. All these pieces of information were obtained after due permission from the Medical Records Section of the hospital. Children on regimen A, aged less than 1 year or more than 18 years, relapse/recurrent cases, and IFIs in the maintenance phase of treatment were all excluded from our study.
Invasive Fungal Infection Prophylaxis
In our unit, AFP was started as per routine practice on 1st July 2017 for those children receiving treatment on regimens B and C of the UKALL 2003 protocol. The prophylaxis was based on the observation of presumed increased incidence of IFI in our cohort of children with ALL. Hence, the study population was divided into two groups, the first group included the children diagnosed before 1st July 2017 and not on AFP and the second group included children diagnosed after 1st July 2017 and on AFP. The antifungal prophylactic agent used was oral voriconazole (dose ranging between 6 to 10 mg/kg/dose) twice daily, and for those who cannot take the drug orally or due to financial constraints, these children received intravenous (IV) conventional amphotericin B (1 mg/kg/d) on alternate days. While receiving amphotericin B, creatinine and potassium levels were monitored twice weekly. Due to the culture pattern in our set-up being fluconazole resistant as well to target molds, voriconazole was chosen as our AFP of choice and the antifungal policy was gradually adjusted. Itraconazole (5 mg/kg/d in two divided doses) and fluconazole (12 mg/kg/dose once-daily dosing) were used as AFP before the introduction of voriconazole in 2017. Unfortunately, therapeutic drug monitoring for voriconazole was not performed on our patients due to the nonavailability of the facility.
Treatment Regimen for Underlying Leukemia
ALL was diagnosed based on bone marrow examination showing blast cells ≥20%-and confirmed through flow cytometry.[12] All the children with ALL were treated on the uniform protocol mentioned in UKALL 2003 as outlined in [Fig. 1].
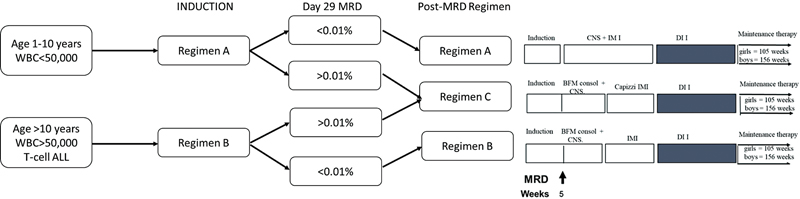
| Figure 1:UKALL 03 treatment regimens. BFM, Berlin–Franklin–Munster consolidation; IM, interim maintenance; DI, delayed intensification; MRD, minimal residual disease.
Treatment for Invasive Fungal Infection
For all children who developed fever, before the initiation of broad-spectrum antibiotics, tests such as blood culture and complete blood count were performed along with urine culture performed in children <5 xss=removed>3) lasting more than 96 hours after starting antibiotics with negative bacterial cultures. If suspicion of IFI was raised, based on prolonged fever and negative cultures, chest X-ray and abdominal ultrasonography were performed in all children. Serum galactomannan levels were analyzed using Platelia Aspergillus Ag enzyme-linked immunosorbent assay kit, and cerebrospinal fluid analysis and culture, computed tomography (CT) chest/sinus, echocardiography, and other imaging studies were performed on a case-to-case basis. IFI was classified according to the European Organization of Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG) guidelines.[13]
Sample Size and Outcome Measures
This was a time-bound study analyzing all the ALL diagnosed children admitted during the study period and including only those satisfying the inclusion criteria. The primary outcome of the study was to identify and classify fungal infections and to assess the role of AFP in preventing them. The secondary outcome measure was to identify the relevant risk factors contributing to the fungal infection.
Ethics
The study was approved by the Institute's Ethics Committee (IEC KMC MLR 02-2021/71), and all the patients consented to the collection and analysis of the data. Kasturba Medical College Hospital, Mangalore, is a tertiary-level teaching hospital that has a dedicated Division of Pediatric Oncology under the Department of Pediatrics. Written informed consent was waived off due to the retrospective nature of the study. No harm was done to the study participants, and all the ethical principles under the Declaration of Helsinki were met.
Definitions
Febrile Neutropenia
A single spike of fever >38°C or 100°F with an absolute neutrophil count lower than 500/mm3, according to National Institute for Health and Clinical Excellence guidance.[14] [15]
Invasive Fungal Infections
EORTC/MSG[13] has standardized the pathological characteristics of proven/probable/possible fungal infection based on host factors, clinical criteria, and mycological criteria.[9] They are as follows:
Possible IFI—the absence of mycological evidence but the presence of both clinical and host factors.
Probable IFI—the presence of all criteria: imaging studies showing features suggestive of fungal infection and mycological evidence of fungal elements from sputum, bronchoalveolar lavage, sinus aspirate using cytology/direct microscopy/culture, or detection of antigen/cell wall constituents (such as beta-galactomannan and beta-glucan).
Proven IFI—histopathological/cytopathologic/microscopic evidence from normally sterile sites showing the fungal organism with evidence of tissue destruction or blood culture growth of a fungal organism.
Statistical Analysis
The data were coded in an excel sheet and fed into IBM Statistical Package for the Social Sciences version 25.0, Armonk, NY, United States for analysis. Frequency and percentage were used to express categorical variables, and continuous variables were expressed with mean and standard deviation. The groups were compared using one-way analysis of variance test to state their significance. The relationship between AFP and invasive fungal disease was tested using binary logistic regression. The associations of IFI with risk factors were analyzed using the chi-square test. p-Value < 0.05 was considered statistically significant in a two-tailed test.
Results
Study Population, Patient Characteristics, and Overall Invasive Fungal Infection
A total of 55 out of 80 NCI high-risk ALL children fulfilled the inclusion criteria ([Fig. 2]). For the rest of the children, either information was inadequate or they were transferred to other centers for management soon after the diagnosis. Among 55 children, 41 children had a high WBC count of ≥50,000/mm3, 12 children had T cell ALL, and 2 children were aged above 10-year-old with a WBC count of <50 xss=removed>3.
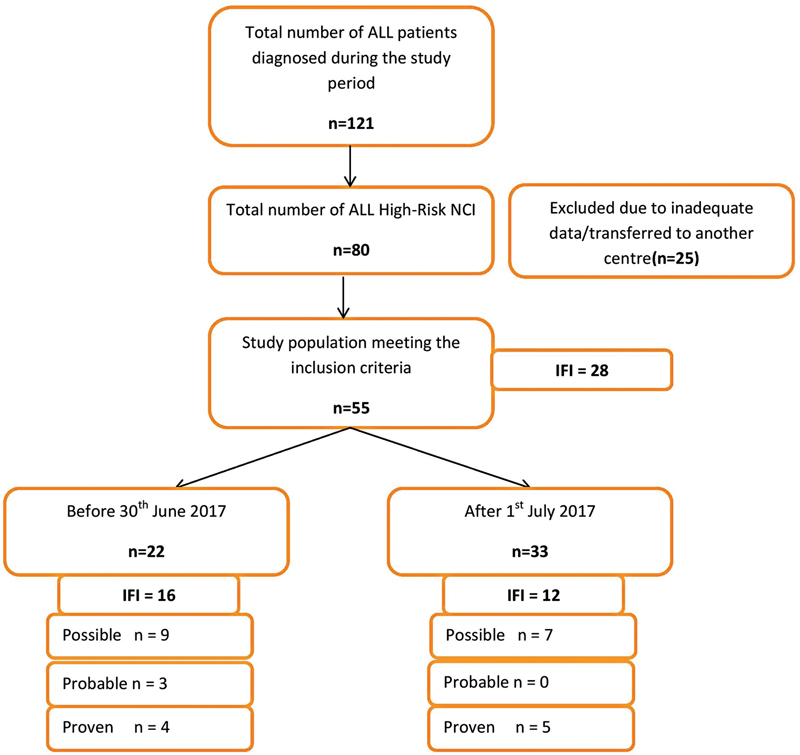
| Figure 2:Flow diagram depicting the study design.
Children were almost equally distributed among regimens B and C following induction (26 and 29, respectively). In our cohort, the total incidence of IFI was 51%- (28/55). Out of the 55 children, 33 (60%) of them were on AFP, and among them, only 12 (28%) had a fungal infection ([Table 1]). Out of 22 children (40%) who were not on AFP, 16 of them developed a fungal infection (70%; p-Value = 0.008).
|
Total number of patients diagnosed with high-risk ALL during the study period |
55 |
|
|
Age |
1–10 y |
38 (69%) |
|
≥10 y |
17 (31%) |
|
|
Sex |
Male |
36 (65%) |
|
Female |
19 (35%) |
|
|
UKALL 03 regimen |
B |
26 (53%) |
|
C |
29 (47%) |
|
|
No. of children on AFP |
33 (60%) |
|
|
No. of children with fungal infection on AFP |
12 (33%) |
|
|
No. of children without AFP |
22 (40%) |
|
|
No. of children with fungal infection not on AFP |
16 (73%) |
|
|
Total number of children with fungal infection |
28 (51%) |
p-Value |
||
|---|---|---|---|---|
|
Age |
1–10 y |
19 (68%) |
0.012 |
|
|
>10 y |
9 (32%) |
|||
|
Sex |
Male |
15 (54%) |
0.706 |
|
|
Female |
13 (46%) |
|||
|
UKALL 03 regimen |
B |
10 (36%) |
0.136 |
|
|
C |
18 (64%) |
|||
|
Central line |
Present |
7 (25%) |
0.012 |
|
|
Absent |
21 (75%) |
|||
|
Type |
Chemoport |
5 (71%) |
||
|
Femoral |
2 (29%) |
|||
|
Phase |
Induction |
14 (50%) |
||
|
Consolidation |
7 (25%) |
|||
|
Delayed intensification |
7 (25%) |
|||
|
IFI |
Possible |
16 (57%) |
||
|
Probable |
3 (11%) |
|||
|
Proven |
9 (32%) |
|||
|
Investigations |
Chest X-ray |
28 |
||
|
Blood culture |
28 |
|||
|
Candida Albicans |
2 (22%) |
|||
|
Candida parapsilosis |
4 (44%) |
|||
|
Candida krusei |
1 (12%) |
|||
|
Candida Tropicalis |
4 (44%) |
|||
|
Galactomannan level (Aspergillus) |
3/3 |
|||
|
Imaging |
USG abdomen |
2 |
||
|
CT sinuses |
2 |
|||
|
CT/MRI head |
3 |
|||
|
CT chest |
2 |
|||
|
2D Echo |
15 (54%) |
|||
|
Variable |
Odd's ratio with 95% confidence interval |
p-Value |
|---|---|---|
|
Gender |
0.33 (0.1–1.1) |
0.054 |
|
Central line |
0.79 (0.24–2.6) |
0.70 |
|
Associated bacterial infection |
– |
0.04 |
|
Age |
1.8 (0.6–5.6) |
0.083 |
|
Final regimen |
1.25 (0.43–3.61) |
0.68 |
|
Author (Ref) |
ALL-HR (Y/N) |
IFI incidence in ALL |
Time period |
Cases (N) |
Mortality |
AFP (A/P) |
Type of study |
Analysis |
|---|---|---|---|---|---|---|---|---|
|
Kumar et al[37] |
N |
14/17 (74.6%) |
2013–2014 |
59 |
4/7(57%) in the induction phase |
A |
Prospective study—New Delhi |
Prevalence of IFI is very high in children with persistent febrile neutropenia who are not on AFP. |
|
Tüfekçi et al[38] |
Y |
7/17 (41%) |
2001–2013 |
174 |
NR |
A |
Retrospective study—Turkey |
Higher prevalence of IFI with persistent febrile neutropenia in HR-ALL children. |
|
Evim et al[39] |
Y |
84/238 (35.2%) with 18 (21%) in HR blocks |
2010–2015 |
238/289 |
34% |
P-26/87 developed on IFI—fluconazole followed by Itraconazole |
Retrospective study—Turkey |
Increased IFI in high-risk ALL children even on AFP and higher mortality rate. |
|
Kaya et al[19] |
N |
10/106 (10.2%) |
1998–2007 |
106/154 |
5% |
P |
Retrospective study—Turkey |
AFP with fluconazole may be reducing the incidence and mortality of IFI. |
|
Supatharawanich et al[40] |
N |
12/150 (8%) |
2009–2019 |
150/241 |
8.3% |
P-4/12 had IFI on AFP (itraconazole and posaconazole) |
Retrospective study—Thailand |
AFP reduces IFI in relapsed leukemia but not in ALL children. |
|
Yi et al[41] |
N |
65/214 (30.7%) |
2014–2017 |
214 |
NR |
A |
Retrospective study—PR China |
The occurrence of IFI in children with ALL relates to the time of hospitalization and the level of neutrophils. |
|
Zhang et al[42] |
Y |
63/155 (40.6%) |
2017–2018 |
155 |
NR |
P-45% IFI—No AFP vs. 37% on AFP (posaconazole and fluconazole) |
Retrospective study—PR China |
Incidence of IFI with AFP was comparable between the two groups (on AFP vs. off AFP). |
|
Das et al[18] |
N |
46/55 (83%) |
2006–2013 |
692 |
44% |
A |
Retrospective study—India |
IFI most common cause of treated related mortality in pediatric ALL. |
|
Bal et al[43] |
N |
24/125 |
2005–2013 |
125 |
13.3% |
A |
Retrospective study—Turkey |
Younger age, prolonged neutropenia, and induction phase chemotherapy were considered risk factors for IFI. |
References
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med 2015; 373 (16) 1541-1552
- Howlader N, Noone AM, Krapcho M. et al: SEER Cancer Statistics Review (CSR) 1975–2013. Bethesda, MD: National Cancer Institute; 2015
- Vora AJ, Goulden N, Mitchell CD. et al. UKALL 2003, a randomised trial investigating treatment intensification for children and young adults with minimal residual disease defined high risk acute lymphoblastic leukaema. Blood 2012; 120 (21) 136
- Vora AJ, Mitchell C, Goulden N. et al. UKALL 2003, a randomised trial investigating treatment reduction for children and young adults with minimal residual disease defined low risk acute lymphoblastic leukaemia. Blood 2010; 116 (21) 496
- Lashkari HP, Faheem M, Hanaganahalli BS. et al. Resource limited centres can deliver treatment for children with acute lymphoblastic leukaemia with risk-stratified minimal residual disease based UKALL 2003 protocol with no modification and a good outcome. Expert Rev Hematol 2020; 13 (10) 1143-1151
- Arora RS, Arora B. Acute leukemia in children: a review of the current Indian data. South Asian J Cancer 2016; 5 (03) 155-160
- Lehrnbecher T, Schöning S, Poyer F. et al. Incidence and outcome of invasive fungal diseases in children with hematological malignancies and/or allogeneic hematopoietic stem cell transplantation: results of a prospective multicenter study. Front Microbiol 2019; 10 (MAR): 681
- Science M, Robinson PD, MacDonald T, Rassekh SR, Dupuis LL, Sung L. Guideline for primary antifungal prophylaxis for pediatric patients with cancer or hematopoietic stem cell transplant recipients. Pediatr Blood Cancer 2014; 61 (03) 393-400
- Castagnola E, Cesaro S, Dalle JH, Engelhard S, Hope W, Lehrnbecher T, Roilides E, Styczynski J, Warris A. ECIL 4–Pediatric Group Considerations for Fungal Diseases and Antifungal Treatment in Children. https://www.leukemia-net.org/treat_research/supportive_care/standards_sop_and_recommendations/e4702/infoboxContent9667/ECIL42011PaediatricguidelinesFungiandantifungals.pdf (last accessed 16 sept 2022)
- O'Connor D, Bate J, Wade R. et al. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood 2014; 124 (07) 1056-1061
- Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am 2015; 62 (01) 61-73
- Vora A, Goulden N, Mitchell C. et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol 2014; 15 (08) 809-818
- De Pauw B, Walsh TJ, Donnelly JP. et al; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46 (12) 1813-1821
- Davis K, Wilson S, Combes A. et al. Febrile neutropenia in paediatric oncology. Paediatr Child Health (Oxford) 2020; 30 (03) 93-97
- NICE. Neutropenic sepsis: prevention and management in people with cancer. NICE guideline. 2012; (September 2012):1–31
- Chang SM, Holt M, Hernandez L. et al. Incidence of invasive fungal infections (IFI) in pediatric acute lymphoblastic leukemia (ALL) and the impact of antifungal prophylaxis in an endemic area. Blood 2021; 138 (Supplement 1): 1215-1215
- Shliakhtsitsava K, Grapsy J, Hsu C. et al. Invasive fungal infections in pediatric patients with high-risk acute lymphoblastic leukemia during initial phases of therapy: a retrospective evaluation. Blood 2020; 136 (Supplement 1): 4-5
- Das A, Oberoi S, Trehan A. et al. Invasive fungal disease in pediatric acute leukemia in the nontransplant setting: 8 years' experience from a tertiary care center in North India. J Pediatr Hematol Oncol 2018; 40 (06) 462-467
- Kaya Z, Gursel T, Kocak U, Aral YZ, Kalkanci A, Albayrak M. Invasive fungal infections in pediatric leukemia patients receiving fluconazole prophylaxis. Pediatr Blood Cancer 2009; 52 (04) 470-475
- Ansari Sh, Shirzadi E, Elahi M. The prevalence of fungal infections in children with hematologic malignancy in Ali-Asghar Children Hospital between 2005 and 2010. Iran J Ped Hematol Oncol 2015; 5 (01) 1-10
- Fisher BT, Robinson PD, Lehrnbecher T. et al. Risk factors for invasive fungal disease in pediatric cancer and hematopoietic stem cell transplantation: a systematic review. J Pediatric Infect Dis Soc 2018; 7 (03) 191-198
- Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc 2017; 6 (1, suppl_1): S3-S11
- Wang SS, Kotecha RS, Bernard A. et al. Invasive fungal infections in children with acute lymphoblastic leukaemia: results from four Australian centres, 2003-2013. Pediatr Blood Cancer 2019; 66 (10) e27915
- Kobayashi R, Kaneda M, Sato T, Ichikawa M, Suzuki D, Ariga T. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: a 10-year analysis at a single institution at Japan. J Pediatr Hematol Oncol 2008; 30 (12) 886-890
- Hale KA, Shaw PJ, Dalla-Pozza L, MacIntyre CR, Isaacs D, Sorrell TC. Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br J Haematol 2010; 149 (02) 263-272
- Lamoth F. Galactomannan and 1,3-β-d-glucan testing for the diagnosis of invasive aspergillosis. J Fungi (Basel) 2016; 2 (03) 22
- Pana ZD, Kourti M, Vikelouda K. et al. Voriconazole antifungal prophylaxis in children with malignancies: a nationwide study. J Pediatr Hematol Oncol 2018; 40 (01) 22-26
- Dvorak CC, Fisher BT, Sung L. et al. Antifungal prophylaxis in pediatric hematology/oncology: new choices and new data. Pediatr Blood Cancer 2012; 59 (01) 21-26
- Mandhaniya S, Swaroop C, Thulkar S. et al. Oral voriconazole versus intravenous low dose amphotericin B for primary antifungal prophylaxis in pediatric acute leukemia induction: a prospective, randomized, clinical study. J Pediatr Hematol Oncol 2011; 33 (08) e333-e341
- Moriyama B, Henning SA, Leung J. et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012; 55 (04) 290-297
- Morris SK, Allen UD, Gupta S, Richardson SE. Breakthrough filamentous fungal infections in pediatric hematopoetic stem cell transplant and oncology patients receiving caspofungin. Can J Infect Dis Med Microbiol 2012; 23 (04) 179-182
- Long S. Incidence of breakthrough invasive fungal infections while on micafungin for antifungal prophylaxis in pediatric hematopoietic cell transplant patients. Biol Blood Marrow Transplant 2020; 26 (03) S387
- Madney Y, Arafah O, Elmahalawy H, Shalby L. Efficacy of voriconazole prophylaxis in pediatric patients with acute myeloid leukemia, single center experience, Egypt. J Leuk (Los Angel) 2019; 07 (02) 1-6
- Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi (Basel) 2017; 3 (04) E57
- Vallabhaneni S, Mody RK, Walker T, Chiller T. The global burden of fungal diseases. Infect Dis Clin North Am 2016; 30 (01) 1-11
- Lehrnbecher T, Fisher BT, Phillips B. et al. Clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with cancer and hematopoietic stem-cell transplantation recipients. J Clin Oncol 2020; 38 (27) 3205-3216
- Kumar J, Singh A, Seth R, Xess I, Jana M, Kabra SK. Prevalence and predictors of invasive fungal infections in children with persistent febrile neutropenia treated for acute leukemia—a prospective study. Indian J Pediatr 2018; 85 (12) 1090-1095
- Tüfekçi Ö, Bengoa ŞY, Yenigürbüz FD. et al. Management of invasive fungal infections in pediatric acute leukemia and the appropriate time for restarting chemotherapy. Turk J Haematol 2015; 32 (04) 329-337
- Evim MS, Tüfekçi Ö, Baytan B. et al. Invasive fungal infections in children with leukemia: clinical features and prognosis. Turk J Haematol 2022; 39 (02) 94-102
- Supatharawanich S, Narkbunnam N, Vathana N. et al. Invasive fungal diseases in children with acute leukemia and severe aplastic anemia. Mediterr J Hematol Infect Dis 2021; 13 (01) e2021039
- Yi XL, Mao Q, Jiang Y, Guo XB, Chen Y. [Treatment of invasive fungal infection in childhood acute lymphoblastic leukemia with amphotericin B and voriconazole]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017; 25 (06) 1627-1630
- Zhang T, Bai J, Huang M. et al. Posaconazole and fluconazole prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. J Microbiol Immunol Infect 2021; 54 (06) 1139-1146
- Bal ZS, Karapinar DY, Karadas N. et al. Proven and probable invasive fungal infections in children with acute lymphoblastic leukaemia: results from an university hospital, 2005-2013. Mycoses 2015; 58 (04) 225-232
Address for correspondence
Publication History
Article published online:
28 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:UKALL 03 treatment regimens. BFM, Berlin–Franklin–Munster consolidation; IM, interim maintenance; DI, delayed intensification; MRD, minimal residual disease.

| Figure 2:Flow diagram depicting the study design.
References
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med 2015; 373 (16) 1541-1552
- Howlader N, Noone AM, Krapcho M. et al: SEER Cancer Statistics Review (CSR) 1975–2013. Bethesda, MD: National Cancer Institute; 2015
- Vora AJ, Goulden N, Mitchell CD. et al. UKALL 2003, a randomised trial investigating treatment intensification for children and young adults with minimal residual disease defined high risk acute lymphoblastic leukaema. Blood 2012; 120 (21) 136
- Vora AJ, Mitchell C, Goulden N. et al. UKALL 2003, a randomised trial investigating treatment reduction for children and young adults with minimal residual disease defined low risk acute lymphoblastic leukaemia. Blood 2010; 116 (21) 496
- Lashkari HP, Faheem M, Hanaganahalli BS. et al. Resource limited centres can deliver treatment for children with acute lymphoblastic leukaemia with risk-stratified minimal residual disease based UKALL 2003 protocol with no modification and a good outcome. Expert Rev Hematol 2020; 13 (10) 1143-1151
- Arora RS, Arora B. Acute leukemia in children: a review of the current Indian data. South Asian J Cancer 2016; 5 (03) 155-160
- Lehrnbecher T, Schöning S, Poyer F. et al. Incidence and outcome of invasive fungal diseases in children with hematological malignancies and/or allogeneic hematopoietic stem cell transplantation: results of a prospective multicenter study. Front Microbiol 2019; 10 (MAR): 681
- Science M, Robinson PD, MacDonald T, Rassekh SR, Dupuis LL, Sung L. Guideline for primary antifungal prophylaxis for pediatric patients with cancer or hematopoietic stem cell transplant recipients. Pediatr Blood Cancer 2014; 61 (03) 393-400
- Castagnola E, Cesaro S, Dalle JH, Engelhard S, Hope W, Lehrnbecher T, Roilides E, Styczynski J, Warris A. ECIL 4–Pediatric Group Considerations for Fungal Diseases and Antifungal Treatment in Children. https://www.leukemia-net.org/treat_research/supportive_care/standards_sop_and_recommendations/e4702/infoboxContent9667/ECIL42011PaediatricguidelinesFungiandantifungals.pdf (last accessed 16 sept 2022)
- O'Connor D, Bate J, Wade R. et al. Infection-related mortality in children with acute lymphoblastic leukemia: an analysis of infectious deaths on UKALL2003. Blood 2014; 124 (07) 1056-1061
- Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am 2015; 62 (01) 61-73
- Vora A, Goulden N, Mitchell C. et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol 2014; 15 (08) 809-818
- De Pauw B, Walsh TJ, Donnelly JP. et al; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46 (12) 1813-1821
- Davis K, Wilson S, Combes A. et al. Febrile neutropenia in paediatric oncology. Paediatr Child Health (Oxford) 2020; 30 (03) 93-97
- NICE. Neutropenic sepsis: prevention and management in people with cancer. NICE guideline. 2012; (September 2012):1–31
- Chang SM, Holt M, Hernandez L. et al. Incidence of invasive fungal infections (IFI) in pediatric acute lymphoblastic leukemia (ALL) and the impact of antifungal prophylaxis in an endemic area. Blood 2021; 138 (Supplement 1): 1215-1215
- Shliakhtsitsava K, Grapsy J, Hsu C. et al. Invasive fungal infections in pediatric patients with high-risk acute lymphoblastic leukemia during initial phases of therapy: a retrospective evaluation. Blood 2020; 136 (Supplement 1): 4-5
- Das A, Oberoi S, Trehan A. et al. Invasive fungal disease in pediatric acute leukemia in the nontransplant setting: 8 years' experience from a tertiary care center in North India. J Pediatr Hematol Oncol 2018; 40 (06) 462-467
- Kaya Z, Gursel T, Kocak U, Aral YZ, Kalkanci A, Albayrak M. Invasive fungal infections in pediatric leukemia patients receiving fluconazole prophylaxis. Pediatr Blood Cancer 2009; 52 (04) 470-475
- Ansari Sh, Shirzadi E, Elahi M. The prevalence of fungal infections in children with hematologic malignancy in Ali-Asghar Children Hospital between 2005 and 2010. Iran J Ped Hematol Oncol 2015; 5 (01) 1-10
- Fisher BT, Robinson PD, Lehrnbecher T. et al. Risk factors for invasive fungal disease in pediatric cancer and hematopoietic stem cell transplantation: a systematic review. J Pediatric Infect Dis Soc 2018; 7 (03) 191-198
- Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc 2017; 6 (1, suppl_1): S3-S11
- Wang SS, Kotecha RS, Bernard A. et al. Invasive fungal infections in children with acute lymphoblastic leukaemia: results from four Australian centres, 2003-2013. Pediatr Blood Cancer 2019; 66 (10) e27915
- Kobayashi R, Kaneda M, Sato T, Ichikawa M, Suzuki D, Ariga T. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: a 10-year analysis at a single institution at Japan. J Pediatr Hematol Oncol 2008; 30 (12) 886-890
- Hale KA, Shaw PJ, Dalla-Pozza L, MacIntyre CR, Isaacs D, Sorrell TC. Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br J Haematol 2010; 149 (02) 263-272
- Lamoth F. Galactomannan and 1,3-β-d-glucan testing for the diagnosis of invasive aspergillosis. J Fungi (Basel) 2016; 2 (03) 22
- Pana ZD, Kourti M, Vikelouda K. et al. Voriconazole antifungal prophylaxis in children with malignancies: a nationwide study. J Pediatr Hematol Oncol 2018; 40 (01) 22-26
- Dvorak CC, Fisher BT, Sung L. et al. Antifungal prophylaxis in pediatric hematology/oncology: new choices and new data. Pediatr Blood Cancer 2012; 59 (01) 21-26
- Mandhaniya S, Swaroop C, Thulkar S. et al. Oral voriconazole versus intravenous low dose amphotericin B for primary antifungal prophylaxis in pediatric acute leukemia induction: a prospective, randomized, clinical study. J Pediatr Hematol Oncol 2011; 33 (08) e333-e341
- Moriyama B, Henning SA, Leung J. et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012; 55 (04) 290-297
- Morris SK, Allen UD, Gupta S, Richardson SE. Breakthrough filamentous fungal infections in pediatric hematopoetic stem cell transplant and oncology patients receiving caspofungin. Can J Infect Dis Med Microbiol 2012; 23 (04) 179-182
- Long S. Incidence of breakthrough invasive fungal infections while on micafungin for antifungal prophylaxis in pediatric hematopoietic cell transplant patients. Biol Blood Marrow Transplant 2020; 26 (03) S387
- Madney Y, Arafah O, Elmahalawy H, Shalby L. Efficacy of voriconazole prophylaxis in pediatric patients with acute myeloid leukemia, single center experience, Egypt. J Leuk (Los Angel) 2019; 07 (02) 1-6
- Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi (Basel) 2017; 3 (04) E57
- Vallabhaneni S, Mody RK, Walker T, Chiller T. The global burden of fungal diseases. Infect Dis Clin North Am 2016; 30 (01) 1-11
- Lehrnbecher T, Fisher BT, Phillips B. et al. Clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with cancer and hematopoietic stem-cell transplantation recipients. J Clin Oncol 2020; 38 (27) 3205-3216
- Kumar J, Singh A, Seth R, Xess I, Jana M, Kabra SK. Prevalence and predictors of invasive fungal infections in children with persistent febrile neutropenia treated for acute leukemia—a prospective study. Indian J Pediatr 2018; 85 (12) 1090-1095
- Tüfekçi Ö, Bengoa ŞY, Yenigürbüz FD. et al. Management of invasive fungal infections in pediatric acute leukemia and the appropriate time for restarting chemotherapy. Turk J Haematol 2015; 32 (04) 329-337
- Evim MS, Tüfekçi Ö, Baytan B. et al. Invasive fungal infections in children with leukemia: clinical features and prognosis. Turk J Haematol 2022; 39 (02) 94-102
- Supatharawanich S, Narkbunnam N, Vathana N. et al. Invasive fungal diseases in children with acute leukemia and severe aplastic anemia. Mediterr J Hematol Infect Dis 2021; 13 (01) e2021039
- Yi XL, Mao Q, Jiang Y, Guo XB, Chen Y. [Treatment of invasive fungal infection in childhood acute lymphoblastic leukemia with amphotericin B and voriconazole]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017; 25 (06) 1627-1630
- Zhang T, Bai J, Huang M. et al. Posaconazole and fluconazole prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. J Microbiol Immunol Infect 2021; 54 (06) 1139-1146
- Bal ZS, Karapinar DY, Karadas N. et al. Proven and probable invasive fungal infections in children with acute lymphoblastic leukaemia: results from an university hospital, 2005-2013. Mycoses 2015; 58 (04) 225-232


 PDF
PDF  Views
Views  Share
Share

