Review of clinical profile and bacterial spectrum and sensitivity patterns of pathogens in febrile neutropenic patients in hematological malignancies: A retrospective analysis from a single center
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(02): 85-88
DOI: DOI: 10.4103/0971-5851.116184
Abstract
Background: The aim of this study was to study clinical profile with bacterial spectrum and susceptibility patterns of pathogens in culture positive febrile neutropenic (FN) patients of hematological malignancies. Materials and Methods: We retrospectively reviewed the medical records of 23 hematological malignancy patients admitted with chemotherapy induced febrile neutropenia with culture positive results, at our institute between June 2011 and December 2011. Results: A total of 23 patients were studied 12 males and 11 females, with a median age of 35 years. Most common diagnosis was acute leukemia (78%) with the majority of patients developing febrile neutropenia during the induction phase of treatment. Most common presenting symptoms were fever, cough, dyspnea, and diarrhea. Primary site of infection was not found in 47% of patients while the rest had lung, gastro-intestinal and skin/soft-tissue infection. Overall 23 organisms were isolated during the study period, from blood (56%), sputum (46%), stool (23%), and nasal swab from one patient. Gram negative bacteria accounted for 78% of organisms while gram positive organisms accounted for 22% of the total isolates. The most common organisms were: Escherichia coli (43%), Staphylococcus aureus (22%), Pseudomonas aeruginosa (17.4%) and Klebsiella pneumonia (17.4%). Antibiotic sensitivity patterns of these bacteria were studied. Gram negative bacterial infections were associated with higher mortality (89%). Conclusions: Induction phase of treatment in acute leukemia is the major cause of FN in hematological malignancies at our institute and gram negative organisms are the predominant organisms with E. coli as major isolate while S. aureus represents the most common gram positive organism. Amikacin and cefoperazone/sulbactum appears to be initial antibiotic appropriate to cover most gram negative pathogens while vancomycin to be added for suspected gram positive infections. FN represents a major cause of morbidity and mortality in hematological malignancy patients, high index of suspicion and early empirical antibiotics with supportive care are main interventions to reduce high mortality for these patients. Antibiotics should be modified according to culture sensitive report as soon as possible.
Publication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
The aim of this study was to study clinical profile with bacterial spectrum and susceptibility patterns of pathogens in culture positive febrile neutropenic (FN) patients of hematological malignancies.
Materials and Methods:
We retrospectively reviewed the medical records of 23 hematological malignancy patients admitted with chemotherapy induced febrile neutropenia with culture positive results, at our institute between June 2011 and December 2011.
Results:
A total of 23 patients were studied 12 males and 11 females, with a median age of 35 years. Most common diagnosis was acute leukemia (78%) with the majority of patients developing febrile neutropenia during the induction phase of treatment. Most common presenting symptoms were fever, cough, dyspnea, and diarrhea. Primary site of infection was not found in 47% of patients while the rest had lung, gastro-intestinal and skin/soft-tissue infection. Overall 23 organisms were isolated during the study period, from blood (56%), sputum (46%), stool (23%), and nasal swab from one patient. Gram negative bacteria accounted for 78% of organisms while gram positive organisms accounted for 22% of the total isolates. The most common organisms were: Escherichia coli (43%), Staphylococcus aureus (22%), Pseudomonas aeruginosa (17.4%) and Klebsiella pneumonia (17.4%). Antibiotic sensitivity patterns of these bacteria were studied. Gram negative bacterial infections were associated with higher mortality (89%).
Conclusions:
Induction phase of treatment in acute leukemia is the major cause of FN in hematological malignancies at our institute and gram negative organisms are the predominant organisms with E. coli as major isolate while S. aureus represents the most common gram positive organism. Amikacin and cefoperazone/sulbactum appears to be initial antibiotic appropriate to cover most gram negative pathogens while vancomycin to be added for suspected gram positive infections. FN represents a major cause of morbidity and mortality in hematological malignancy patients, high index of suspicion and early empirical antibiotics with supportive care are main interventions to reduce high mortality for these patients. Antibiotics should be modified according to culture sensitive report as soon as possible.
INTRODUCTION
Febrile neutropenia (FN) is a common complication of cancer treatment. With the development of more aggressive chemotherapy regimens for hematological malignancies, the survival of these patients has improved. At the same time, these patients frequently succumb to febrile neutropenia, which is the major cause of morbidity and mortality. Studies have reported that 48-60% of patients admitted with febrile neutropenia have an infection, which can be life-threatening as well.[1]
Over the past decade, there has been a considerable change in the spectrum and antibiotic susceptibility patterns of pathogens causing infection in FN patients. Knowledge of locally prevalent pathogens and their sensitivities is essential as it helps to guide antimicrobial therapy in neutropenic patients.[2] The most effective empirical antimicrobial regimen must be rapidly administered to FN patients as delay in the initiation of treatment may result in septicemic shock and thus increase mortality.
To have an insight into the clinical profile of these FN patients as well as spectrum of antimicrobial susceptibility pattern, we have done this retrospective study.
MATERIALS AND METHODS
All clinical and microbiological data were collected retrospectively from FN patients admitted at our institute between June 2011 and December 2011. The inclusion criteria's include hematological malignancy patients having FN and culture positive report. Patient population consisted of both adults and pediatrics with acute and chronic leukemia, myelodysplastic syndrome (MDS), multiple myeloma and other malignancies. FN was defined as fever greater than 38.5°C on one occasion and with an absolute neutrophil count (ANC) of less than 0.5 × 109/L. Cultures were taken from blood in all cases and from urine, stool, tracheal aspirate, sputum or wound depending upon identifiable focus of infection. Blood cultures were processed using the Bactec blood culture system. Organisms were identified according to routine bacteriological procedures. Antibiotic susceptibility testing was performed by disc diffusion method. Results of these were interpreted according to the Clinical Laboratory Standards Institutes guidelines.
RESULTS
A total of 23 (12 males and 11 females) patients with chemotherapy induced FN were analyzed. Table 1 shows the characteristics of these patients. The mean age of the study population was 35 years (range 16-63 years). Majority of patients were of acute leukemia (78%), acute myeloid leukemia (AML) 48% (11/23), acute lymphoblastic leukemia (ALL) 30% (7/23), one patient each of chronic myeloid leukemia (CML), MDS, multiple myeloma, and two patients of other diagnosis. Majority of patients had developed FN during the induction phase of acute leukemia treatment. Most common presenting symptoms were fever, cough, dyspnea and diarrhea.
Table 1
Baseline patients characteristics
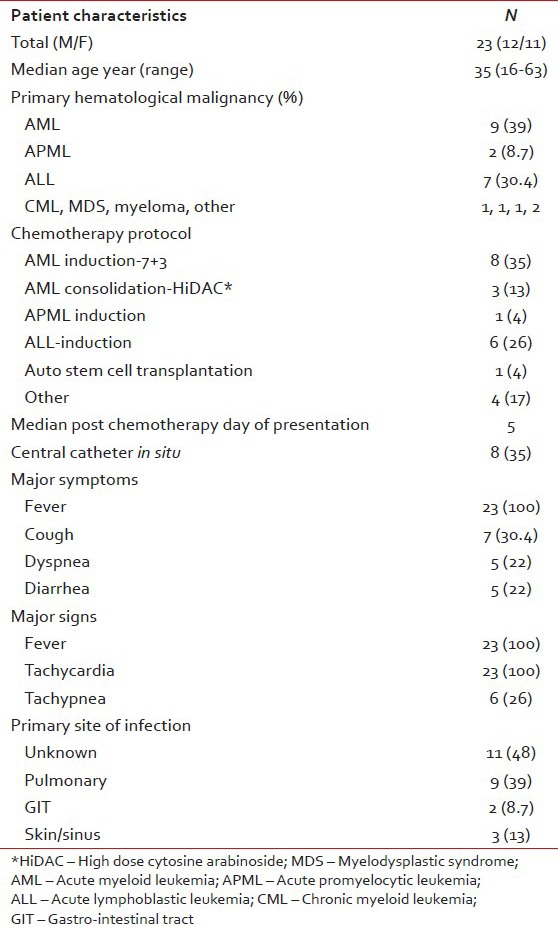
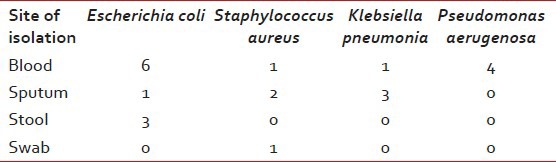
Table 2 shows major laboratory findings. Median ANC was 120/μl, majority of patients also had anemia (median Hb 6.8 g/l) and thrombocytopenia (median platelet count 12000/μl). Primary site of infection was not found in 47% of patients while the rest had lung, gastrointestinal tract and skin/soft-tissue infection. Overall 23 organisms were isolated during the study period, from blood (56%), sputum (46%), stool (23%), and nasal swab from one patient. Table 3 shows various isolated bacteria with respect to the site of isolation. Gram negative bacteria accounted for 78% of organisms while gram positive organisms accounted for 22% of the total isolates. The most common organisms were: Escherichia coli (43%), Staphylococcus aureus (22%), Pseudomonas aeruginosa (17.4%) and Klebsiella pneumonia (17.4%). Figures Figures11 and and22 shows sensitivity and resistance patterns of these four organisms respectively. Majority of E. coli strains were sensitive to amikacin, ciprofloxacin and tigecycline while the majority were resistant to piperacillin/tazobactum (P/T), ceftazidime and cefazolin. P. aeruginosa strains were highly sensitive to amikacin, cefoperazone/sulbactum (C/Su) and ciprofloxacin while they were resistant to tigecycline and tobramycin. For K. pneumonia main sensitive antibiotics were amikacin and tigecycline and resistant antibiotics were P/T, cefazolin and ciprofloxacin. Lastly for S. aureus main sensitive antibiotics were vancomycin, linezolid and gentamicin and resistant one were penicillin, erythromycin and ciprofloxacin.
Table 2
Major investigation findings
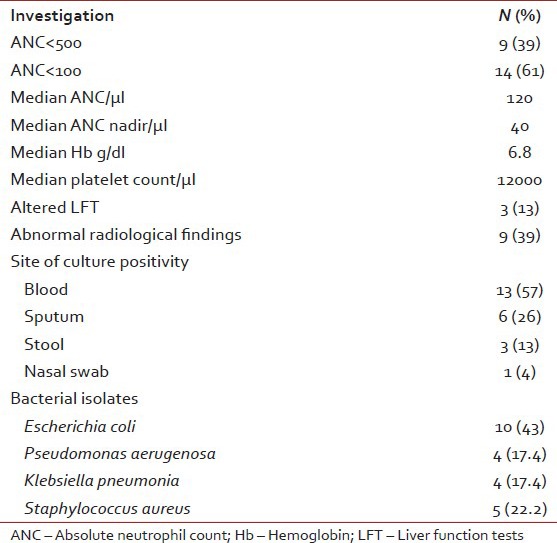
Table 3
Site of culture positive with the organism
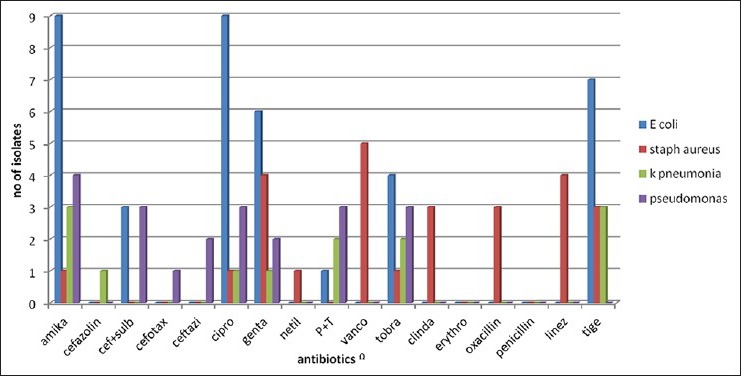
| Fig. 1 Antibiotic sensitivity pattern in bacterial isolates
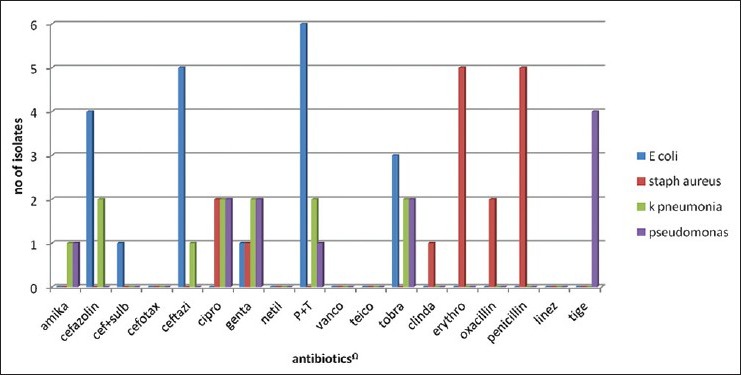
| Fig. 2 Antibiotic resistance pattern in bacterial isolates
Empirical antifungal were used in 15 patients, amphotericin (7), fluconazole (6) and voriconazole (2).
Most common antibiotics used were P/T, amikacin, imipenum/cilastatin (I/C) and C/Su, most were empirically started. Other than antibiotics, patient also received growth factors (7/23) for an average of 11 days, Packed Cell Volume support (15/23) with an average of 5 units and platelet support (15/23) with an average of 17 units. Table 4 shows outcomes of our patients. Median duration of neutropenia was 8.5 days, median duration of hospital stay was 16 days with recovery in 61% and mortality due sepsis in 39%. Gram negative bacterial infections were associated with higher mortality (89%). Table 5 shows mortality in respect to different bacteria.
Table 4
Outcome of patients

Table 5
Mortality with respect to isolated organism

DISCUSSION
Over the past 25 years, there has been a shift in the microbiological spectrum from gram negative to gram positive organisms at many cancer centers.[3] The possible reasons for this shift are better control of gram negative infections with newer ultra-broad spectrum antibiotics, fluoroquinolone prophylaxis, and increase use of long dwelling intravenous devices.[4,5] This study demonstrates that gram negative organisms are still the predominant pathogens causing bacteremia in FN patients. The spectrum of bacterial isolates in our study is similar to what has been reported in both local and international studies. Coagulase negative staphylococci were the most commonly isolated gram positive organisms.[3,6] E. coli was the most frequently isolated gram negative pathogen.[3,7]
This study shows that C/Su and amikacin may represent current best empirical antibiotics for suspected gram negative infections while for gram positive infections; vancomycin and linezolid may play the same role. As soon as the culture sensitivity report comes, antibiotics should be modified for best outcomes. Currently, P/T, amikacin, I/C and C/Su are the most common empirical antibiotics used at our institute.
Our study shows that FN is one of the major causes of morbidity and mortality in patients of hematological malignancies. These patients may not present with any obvious source of infection except blood stream infection. Hence, in all patients suspected of FN, blood culture sensitivity should be sent if possible before the first dose of antibiotics. Furthermore, these patients generally have associated anemia and thrombocytopenia, which should be adequately supported with blood transfusion. Growth factors should be used except in situations like during induction regimens of acute leukemia.
CONCLUSIONS
Induction phase of treatment of acute leukemia are the major cause of FN in hematological malignancies at our institute and gram negative organisms are the predominant organisms with E. coli as major isolate while S. aureus represents the most common gram positive organism. Amikacin and C/Su appears to be initial antibiotic appropriate to cover most gram negative pathogens while vancomycin to be added for suspected gram positive infections. FN represents a major cause of morbidity and mortality in hematological malignancy patients, high index of suspicion and early empirical antibiotics with supportive care are main interventions to reduce high mortality for these patients. Antibiotics should be modified according to culture sensitive report as soon as possible. Continuous surveillance of the spectrum of locally prevalent pathogens and their susceptibility patterns is essential for formulation of therapeutic regimens for chemotherapy induced FN patients.
ACKNOWLEDGMENTS
Dr. Shilin N. Shukla (MD, Hon director Gujarat Cancer and Research Institute Ahmedabad), Dr. Pankaj M. Shah (MD, Ex Director Gujarat Cancer and Research Institute Ahmedabad), Dr. Shailesh S. Talati (MD, Professor Department of Medical and Paediatric Oncology, Gujarat Cancer and Research Institute Ahmedabad), Dr. Asha N. Anand (MD, DM professor Department of Medical and Paediatric Oncology, Gujarat Cancer and Research Institute Ahmedabad), nursing staff and patients of Gujarat Cancer and Research Institute.
Footnotes
Source of Support: Department of Medical and Paediatric Oncology and Department of Microbiology Gujarat Cancer and Research Institute, Ahmedabad
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Antibiotic sensitivity pattern in bacterial isolates

| Fig. 2 Antibiotic resistance pattern in bacterial isolates


 PDF
PDF  Views
Views  Share
Share

