Retrospective Study of B Lymphoblastic Leukemia to Assess the Prevalence of TEL/AML1 in South India: A Study of 214 Cases and Review of Literature
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol
DOI: DOI: 10.1055/s-0042-1742611
Abstract
Introduction?Translocation t(12;21)(p13;q22), a recurrent and an invisible chromosomal abnormality, resulting in?TEL/AML1?gene fusion, associated with good prognosis, has been described to be a common abnormality, in children with B-acute lymphoblastic leukemia (B-ALL).
Objectives?The initial observation of very few TEL/AML1 positive patients at this center on testing by fluorescence in situ hybridization (FISH) led to study the prevalence of the abnormality, compare with the global distribution, and evaluate clinical, pathological, molecular, and cytogenetic features in?TEL/AML1?positive patients.
Materials and Methods?A retrospective study of all B-ALL patients tested for?TEL/AML1?gene fusion during the period January 2009 to November 2020 was undertaken. Clinicopathological, molecular, cytogenetic, treatment, and follow-up details were collected. All publications dealing with?TEL/AML1?gene rearrangement were reviewed post Google and PubMed search.
Results?TEL/AML1gene rearrangement was assessed by FISH in 178 patients and by reverse transcription polymerase chain reaction in 36 patients and detected as the sole abnormality in 8.4% patients with additional genetic abnormalities noted on FISH evaluation. Normal karyotype was noted in 14/18 (77.7%) of these patients and 2 had complex karyotype. Complete blood count revealed hemoglobin to range from 35 to 116 g/L (median: 74 g/L), white blood count: 1.01?110?109/L (median: 7.8?109/L), platelet counts: 10?115?109/L (median: 42?109/L), blast count in peripheral smear: 0?98% (median: 41%). Immunophenotyping demonstrated 94.4% were CD34 positive, common acute lymphoblastic leukemia associated antigen (CALLA) positive with aberrant expression of CD13, CD33, CD56, singly or in combination in 58.8%.
Conclusion?TEL/AML1 fusion is rare in Indian patients with B-ALL and appears to be much rarer in our region. The detection of relevant specific abnormalities is of fundamental importance in B-ALL patients and these geographic variations can be used in defining management policies.
Keywords
TEL/AML1 - t(12;21) fluorescence in situ hybridization - acute lymphoblastic leukemia (ALL) - RT-PCRSource(s) of Support
Nil.
Presentation at a Meeting
None.
Supplementary MaterialPublication History
20 May 2022 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction?Translocation t(12;21)(p13;q22), a recurrent and an invisible chromosomal abnormality, resulting in?TEL/AML1?gene fusion, associated with good prognosis, has been described to be a common abnormality, in children with B-acute lymphoblastic leukemia (B-ALL).
Objectives?The initial observation of very few TEL/AML1 positive patients at this center on testing by fluorescence in situ hybridization (FISH) led to study the prevalence of the abnormality, compare with the global distribution, and evaluate clinical, pathological, molecular, and cytogenetic features in?TEL/AML1?positive patients.
Materials and Methods?A retrospective study of all B-ALL patients tested for?TEL/AML1?gene fusion during the period January 2009 to November 2020 was undertaken. Clinicopathological, molecular, cytogenetic, treatment, and follow-up details were collected. All publications dealing with?TEL/AML1?gene rearrangement were reviewed post Google and PubMed search.
Results?TEL/AML1gene rearrangement was assessed by FISH in 178 patients and by reverse transcription polymerase chain reaction in 36 patients and detected as the sole abnormality in 8.4% patients with additional genetic abnormalities noted on FISH evaluation. Normal karyotype was noted in 14/18 (77.7%) of these patients and 2 had complex karyotype. Complete blood count revealed hemoglobin to range from 35 to 116 g/L (median: 74 g/L), white blood count: 1.01?110?109/L (median: 7.8?109/L), platelet counts: 10?115?109/L (median: 42?109/L), blast count in peripheral smear: 0?98% (median: 41%). Immunophenotyping demonstrated 94.4% were CD34 positive, common acute lymphoblastic leukemia associated antigen (CALLA) positive with aberrant expression of CD13, CD33, CD56, singly or in combination in 58.8%.
Conclusion?TEL/AML1 fusion is rare in Indian patients with B-ALL and appears to be much rarer in our region. The detection of relevant specific abnormalities is of fundamental importance in B-ALL patients and these geographic variations can be used in defining management policies.
Keywords
TEL/AML1 - t(12;21) fluorescence in situ hybridization - acute lymphoblastic leukemia (ALL) - RT-PCR
Introduction
Detection of recurrent genetic abnormalities in hematological malignancies by fluorescence in situ hybridization (FISH) is part of routine standard of care. Recurrent cryptic translocation t(12;21)(p13;q22) in pediatric B-acute lymphoblastic leukemia (B-ALL) is associated with specific clinicopathological characteristics and favorable prognosis.[1] [2] [3] [4] Assessment for?TEL/AML1(ETV6/RUNX1)?fusion enables proper treatment strategies in a given population. Review of online hospital-based reports, international projects, multicenter studies, and review articles[3] [4] [5] demonstrated a wide variation in global incidence, with incidence in west being ?25%[4] and lesser in India ([Table S1] and [S2]-supplementary material).[5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] However, the initial observation of very less number of prognostically favorable TEL/AML1 rearranged B-ALL patients from our tertiary care cancer center in south India prompted focus on evaluating the prevalence of this abnormality. The aim and objectives of the study were to see the prevalence of?TEL/AML1?gene fusion in newly diagnosed B-ALL patients, compare with the global distribution of the fusion gene, and evaluate the clinical, pathological, molecular, and cytogenetic features where available in?TEL/AML1?positive patients.
Materials and Methods
A retrospective study of all cases, previously diagnosed as B-ALL during the period January 2009 to November 2020, at a tertiary care cancer center from South India, was undertaken. Data on demographic, clinical, hematological, interphase FISH, cytogenetics, reverse transcription polymerase chain reaction (RT-PCR) findings, treatment protocol, and follow-up details were collected from the case files in a format. All the newly diagnosed cases for which ALL prognostic panel (BCR-ABL, TEL-AML1, MLL)?was performed and TEL AML result was available were included in the study. All cases where adequate blasts counts were not evident and also relapsed ALL cases were excluded from the study. Primary outcome provides information on the prevalence of TEL/AML1 gene fusion positivity in patients diagnosed with B-ALL from this region and for understanding how the prevalence compares with the global incidence for managing the patients. Secondary outcome includes defining the clinicopathlogic features, cytogenetics and FISH findings, baseline lab values and overall survival in the TEL AML1 positive patients that were analyzed.
FISH: Interphase FISH studies were performed till November 2019, on heparinized bone marrow/peripheral blood fixed samples in 178 patients. LSI ETV6(TEL)/RUNX1(AML1) extra signal dual color translocation probe set and ZytoLightETV6/RUNX1(TEL/AML1)?dual color dual fusion probe were used for testing in 81and 97 samples, respectively. The probe details, typical signal patterns and interpretation are provided in [Table S3] ([Supplementary material]). The denaturation and hybridization procedures were performed according to the manufacturer's instructions and counterstained with 4,6-diamino-2-phenyl-indole stain. Fluorescent signals were visualized at 1250???magnification. The cutoff for TEL/AML1 positivity was 5%. For each case, 200 interphase cells were evaluated by two analysts.
The status of?BCR ABL?fusion and?MLL?gene rearrangement was also evaluated using appropriate probes ([Table S3]).
RT-PCR:?TEL/AML1?mutation was assessed by RT-PCR in 36 patient samples from December 2019 onward. RNA was extracted from bone marrow/peripheral blood samples in ethylenediaminetetraacetic acid, using Qiagen RNA blood mini kit and, c-DNA synthesis and real time PCR was performed using TRU-PCR ALL Panel Kit Version 2.1 (3B Black Biotech India Ltd, Bhopal, India).
Data on karyotype was available in 92 ALL cases. Twenty-four or forty-eight-hour unstimulated heparinized bone marrow/peripheral blood cultures had been set up as per standard protocol. Twenty metaphases were evaluated, and karyotype reported as per International System for Human Cytogenomic Nomenclature (2016).[31] Correlation with cytogenetics was done where available.
Statistical Analysis
Since the number of?TEL/AML1?positive cases were few, simple statistical measure, percentage was used to express the prevalence and other data.
Ethics
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964, as revised in 2013. Patient consent was taken prior to the enrolment of participants in the study. Approval of the study was obtained from Basavatarakam Indo American cancer Hospital & Research Institute's ethical committee. (EC Reference study code: 96, dated 27 May 2021).
Results
The patient cohort consisted of 214 B-ALL patients, at diagnosis, both adults and children. Data on demographic, clinical hematological, FISH, and karyotype findings are presented in [Tables 1], [2], and [3]. A total of 8.4% (18/214) patients harbored the?TEL/AML1?gene rearrangement. The fusion was detected by FISH in 15/178(8.4%) patients and by RT-PCR in 3/36 (8.3%) patients. The clone size or positivity rate varied from 8.9 to 95%. The fusion was frequent in 17/125 (13.6%) children, aged between 2 and 13 years (median: 4 years), and an 18-year-old young adult. Fusion was noted in 94.4% males ([Table 1]).
|
Case no |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age(y) and gender M/F |
5?M |
3?M |
4?M |
4?M |
2?M |
4?M |
4?F |
18 M |
5?M |
2?M |
8?M |
4?M |
13 M |
5?M |
3?M |
8?M |
6?M |
7?M |
|
Clinical presentation and history |
Fever and weakness?2 months |
Fever and weakness?1 week |
Fever?2 weeks and multiple petechial rashes |
Fever?1 week and multiple joint pains |
Fever?2 weeks and joint pain for 1 week |
Fever?1 month, bilateral cervical LN, S+. |
Fever?1 month, cough cold?vomiting petechial rash?1 day.H+S+ |
Fever and AD |
Fever and weakness?1 week. |
Fever?2 weeks and multiple petechial rashes |
Fever?1 month, cough cold, vomiting, petechial rash?1 day. H+S+ |
Fever?1 week and multiple joint pains |
Fever?2 weeks, joint pain?1 week, bilateral cervical LN, S+ |
Fever?1 month bilateral cervical LN,S+ |
Fever and pallor |
Fever?1 week vomitting |
Fever?2weeks Cough cold |
Fever >1 month |
|
HB g/L: |
73 |
62 |
89 |
35 |
69 |
48 |
97 |
85 |
74 |
91 |
116 |
89 |
77 |
74 |
69 |
88 |
76 |
7 |
|
WBCX10(12)/L: |
9.7 |
110 |
3.2 |
36.7 |
5.92 |
4.5 |
6.8 |
17.95 |
4.59 |
1.91 |
6.12 |
3.66 |
8.9 |
1.01 |
50.31 |
17.5 |
11.22 |
23.62 |
|
Platelet x10(9)/L: |
100 |
10 |
45 |
12 |
86 |
17 |
65 |
89 |
22 |
74 |
115 |
24 |
15 |
62 |
18 |
50 |
39 |
45 |
|
PS smear blasts (%) |
25 |
95 |
10 |
90 |
18 |
6 |
50 |
46 |
0 |
30 |
36 |
65 |
4 |
98 |
70 |
56 |
80 |
|
|
BM blasts (%) |
85 |
90 |
77 |
88 |
95 |
60 |
95 |
58 |
80 |
88 |
40 |
69 |
77 |
3 |
55 |
|||
|
PAS |
+ |
+ |
NC |
ND |
+ |
+ |
+ |
N |
G + |
|||||||||
|
B-ALL CD10 +/? |
+ |
+ |
+ |
+ |
? |
+ |
+ |
+ |
+ |
|||||||||
|
Aberrant markers |
CD13 & CD33 |
CD56 |
CD13 & CD33 |
CD13 & CD33 |
CD13 |
CD13 |
||||||||||||
|
TEL AML1 |
*P |
*P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P |
P# |
P# |
P# |
|
Signal pattern |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
AT |
T |
AT |
AT |
AT |
AT |
AT |
|||
|
FISH signal pattern |
1G 2O3F |
2O1F |
1G1O1F |
3G1O1F |
2G2O1F. 2G 1O 1F |
2G1O1F |
2G1O1F |
2G2O2F |
1G1O1F, |
1G1O2F, |
2G2O2F, |
2G1O1F, |
1G1O1F, |
1G1O1F |
2G2O1F, 3G3O |
|||
|
Interpretation |
1 |
2 |
3 |
3?+?4(1-2) |
6(1)?3+ 4(1) +5(1) 6(2)?3+ 4(1) |
3?+?4 (1) |
3+ 4 (1) |
4(1)?+?5(1) |
3 |
4(1)?+?5(1) |
3?+?4(1) |
3 |
3 |
6(1)- P 3+ 4(2) +5(1) 6(2)- N, 4(1) +5(1) |
||||
|
Clone no and size (%) |
39 |
61 |
10 |
89 |
38 20 |
47 |
42 |
83 |
95 |
64 |
91 |
11 |
8.9 |
5 |
P 16 N |
|||
|
BCR-ABL, MLL, E2A-PBX |
N N |
N N |
N N |
N N |
ND,N |
ND, N |
N, N, N |
ND, N |
ND, N loss of MLL ?91% |
ND,N |
ND,N |
#N , N |
ND, MLL -ve |
# N, N |
ND,N |
ND |
ND |
ND |
|
Karyotype |
NK |
NK |
NK |
NK |
IM |
NK |
NK |
NK |
NK |
NK |
NK |
* 46 |
NK |
NK |
NK |
AK |
NM |
AK |
|
Parameters |
Group 1: 1?18 years |
Group 2: 19?39 years |
Group 3: >40 years |
|
|---|---|---|---|---|
|
Overall, no of patients (%) |
125(58.41) |
63(29.43) |
26(12.14) |
|
|
Median age of presentation (y) |
5 |
26 |
46.5 |
|
|
No of males No of females Gender of baby not disclosed |
77 47 1 |
45 18 |
15 11 |
|
|
Male female ratio |
1.6:1 |
2.5:1.0 |
1.3:1 |
|
|
TEL AML1 fusion positive cases |
Total:18/214 (8.41%), FISH:15/178(8.6%) / RT-PCR:3/36(8.3%) |
|||
|
TEL AML1 fusion positive cases as per age group by both technologies: No (%) |
17/125(13.6%) |
1/63(1.58%) |
? |
|
|
TEL AML1 negative cases:196/214(91.58%) |
TELAML1negative with additional genetic findings:39/163(23.9%) |
|||
|
Types of additional abnormalities in negative group No of patients (%) &?lls with abnormal findings in different patients. |
||||
|
Additional copies of AML1?21(12.8%) 24?92?lls 2 patients:1 copy 4patients;2 copies 1 patient;1?2 copies 2 patients:3 copies 3 patients:3?4 copies 4 patients:4 copies 5patients:5 copies |
Additional copies of TELgene?2 patients (1.2%) 43?63?lls?3 copies in each patient |
Additional copies of TEL and AML1genes?8(4.9%) Patient:2 clones seen: Clone 1?44?lls had 4 copies of only AML1. Another clone?34?lls had 3copies of Tel& 4 copies of AML1 Patient: 2 clones seen. Clone 1: 42.3?lls had 3?4 copies of AML1only. Clone 2: 28.46?lls had 3 copies of Tel& 3?4 copies of AML1 genes Patient: 3?4 copies AML1&TEL genes seen in 81?lls Patient: Clone1?50?lls had4 copies of AML1&3 copies of TEL gene 2ndclone?34% had 3 copies of TEL gene Patient: 83?lls had 3 copies TEL gene & 77?lls had 3?7 copies of AML1 Patient: 65?lls had 5 copies TEL& 4?5 copies AML1 gene Patient:40?lls had (2 copies) AML1 gene & 1 copy of TEL gene Patient:18?lls had 1 copy of TEL gene and 2 copies of AML1 gene |
Deletion of TEL gene and amplification of AML12(1.2%) Patient: Loss of TEL gene in all cells and 40?lls?3 copies of AML1 Patient: Loss of TEL gene and 4 copies AML1?59?lls |
Del of TEL gene6(3.7%) 14.5?86.8?lls |
|
Case no |
Cytogenetic findings |
FISH findings |
Correlation of karyotype and FISH findings |
|---|---|---|---|
|
1 |
47,XX,+8[14],46XX[2]* |
TEL/AML1 negative, with amplification of the AML1 gene?2 copies in 45?lls |
Cryptic amplification of AML1 gene |
|
2 |
50?57,XX/XXX,+X[11],+4[10],+5[6],+6[13],+8[6],+9[7],+10[6],+11[8],+12[3],t(?;12)(?;q24.1)[5],+14[4],+15[2],+16[3],+17[12],+18[6],?9[11],+20[3],+21[10][cp16]/46,XX[4] |
TEL/AML1 negative, amplification of AML1 gene (1 copy) |
Karyotype demonstrating a+21 corroborating with FISH amplification. |
|
3 |
46,XY,dup(9)(q34.1q34.1)[4]/47,XY,+mar[2]/46,XY[9] |
TEL/AML1 negative, amplification of AML1 gene1?2 copies -33% |
Cryptic amplification of AML1 seen. |
|
4 |
46,XY,del(8)(p22)[8]/46,XY[6] |
TEL/AML1 negative, Amplification of Tel and AML1 genes |
Cryptic amplification of TEL and AML1 genes |
|
5 |
50?53,XXY/XY,+X,+4,+6,+8,+10,+14,+17,+18, +21,+22[cp15]/46,XY[5] |
TEL/AML1 negative, amplification of AML1 gene?2 copies |
Karyotype shows gain of chromosome 21 (only 1 copy) and the other copy of AML 1 is cryptic. |
|
6 |
54?55,XY,+4[4],+6[2],+8[3],+10[3],+16[1],+17 [3],+17?2[1],+18[3],+21[2],+21?2[2],+22[3][cp4]/46,XY[16]13 |
TEL/AML1 negative, amplification of AML1 gene?2 copies?58% |
Karyotype and FISH demonstrate gain of 2 copies of chromosome 21 and 2 copies of AML1 gene |
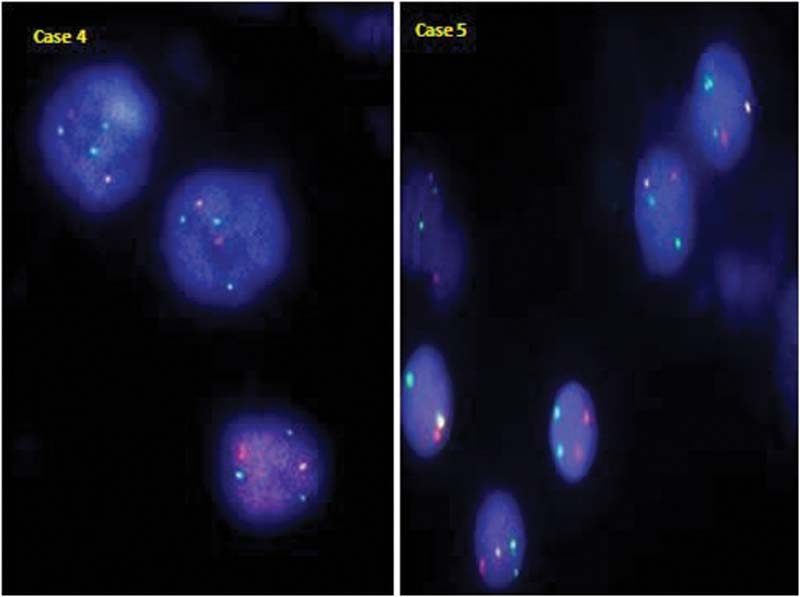
|?Fig. 1TEL/AML1 positive case with an atypical signal pattern showing 1 fusion with additional copies of AML1 (green) and TEL (orange) gene in case 4 and an additional copy of only AML1 gene (green) in case 5 (clone 2).
TEL/AML1?fusion was absent in 91.58?ses. Additional genetic abnormalities were also noted in 23.9% of these patients, with amplification of?AML1, seen more often ([Table 2]).
Cytogenetic analysis in 92 B-ALL patients revealed the karyotype was normal in 57 (61.95%) and abnormal in 35 /92 (38.04%) patients. It is important to note that 14 patients with normal karyotype and cases 16 and 18 with abnormal complex cytogenetics had?TEL/AML1?rearrangement/ mutation. Case 18 showed evidence of del(12)(p13)in 60% metaphases ([Table 1]-footnote).
Correlation of FISH results and cytogenetics demonstrated?TEL/AML1?rearrangement to be the sole abnormality and mutually exclusive as BCR ABL and MLL were negative for rearrangement in all cases wherever done. Additional genetic abnormalities noted in the positive group, occurred when the chromosome complement was 46 with two intact homologous chromosomes 12 and 21 suggesting that the abnormalities were submicroscopic/cryptic level.
Among the 39 cases negative for TEL/AML1 rearrangement but with additional abnormalities, karyotype was normal in 8 and abnormal in 6 cases. Correlation studies demonstrated that the loss or gain of the genes was cryptic in the former. However, in six cases having an abnormal karyotype,?AML1?amplification was cryptic in 50% and corroborated by additional chromosome 21 ([Table 3]).
Complete blood count (CBC) in patients positive for?TEL/AML1fusion revealed low hemoglobin in all patients (range: 35?116?g/L, median: 74?g/L), high white blood cell (WBC) counts in 8/18 (44.4%) and 7/17(41.2%) pediatric patients (range: 11.2?110???109/L) and low counts in 4/18(22.2%) and 4/17 pediatric patients (23.52%) (range: 1.01?3.6). Platelet counts were low in all patients (range: 10?115???109/L, median: 42???109/L). The blasts ranged from 0 to 98% (median: 41%) in peripheral smear and 0 to 95% (median: 80%) in the bone marrow ([Fig. 2]). Immunophenotyping revealed patients had CD34 positive phenotype, common acute lymphoblstic leukemia associated antigen (CALLA) positive in 17/18 (94.4%) and aberrant expression of CD13, CD33, CD56, singly or in combination in 10/17(58.8%) patients. Coexpression of CD13 and CD33 occurred in 70% ([Fig. 3]), only CD3 in 20%, and only CD56 in 10%.
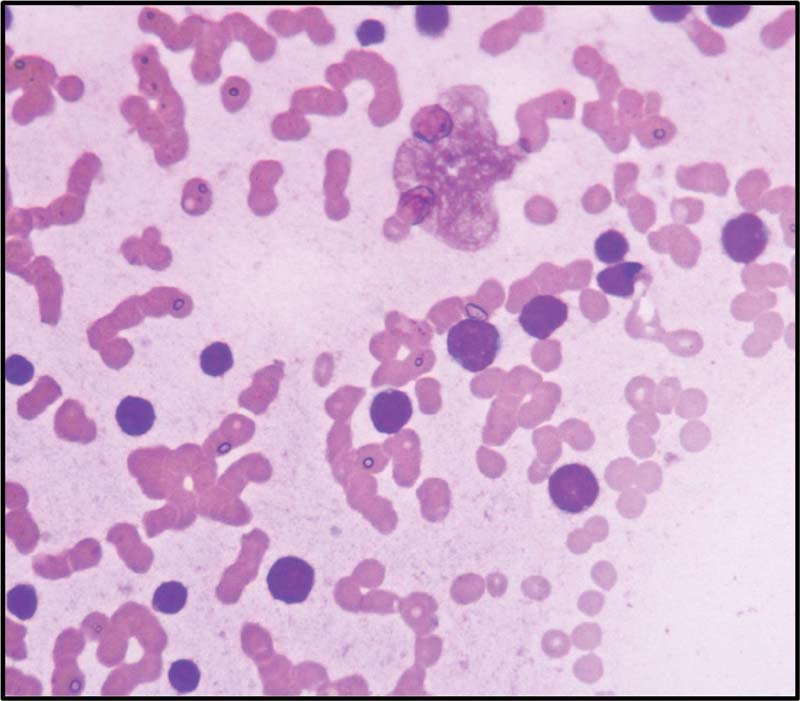
|?Fig. 2Blasts showing high N/C ratio coarse chromatin and scanty cytoplasm (Leishman stain,400x).
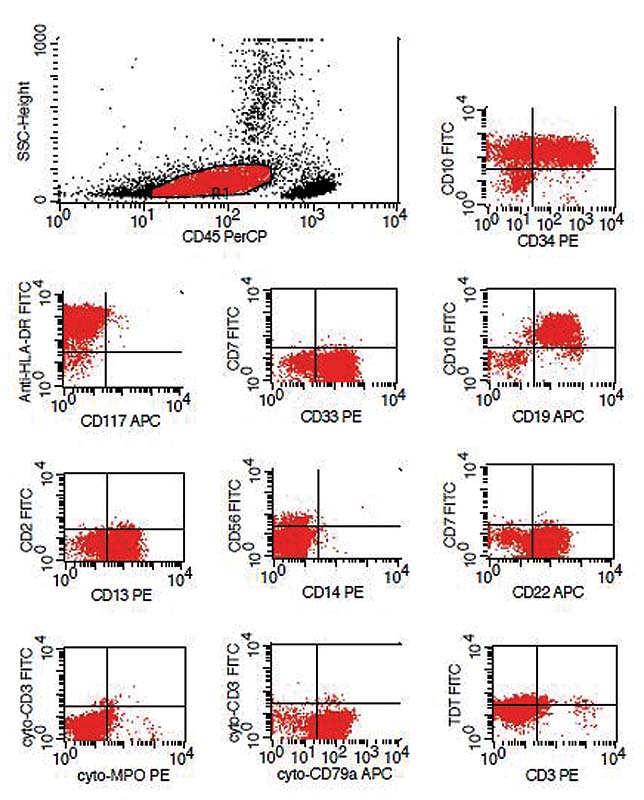
|?Fig. 3Immunophenotyping in a prototype case showing aberrant expression of CD13 and CD33.
Treatment and follow-up details: All the 18 patients were treated with ALL-Berlin-Frankfurt-Munster (BFM 95) protocol. One child died after 1 month due to thrombotic complication. Three patients completed induction and consolidation and lost to follow-up. Fourteen patients who completed the whole treatment are alive and disease free. The median survival among those who completed treatment is 35.5 months (range: 19?44 months).
Discussion
ALL is the most frequent malignancy accounting for 85% of all childhood leukaemia.[32] [33] [34] [35] [36] B-ALL constituted 76% of all ALL that comprised of predominantly CALLA positive ALL in 73%, early precursor pro B-ALL in 3%.[33] The World Health Organization classification of hematopoietic and lymphoid tissue (2017) defines B lymphoblastic leukemia/lymphoma into various subtypes including ?B lymphoblastic leukemia/lymphoma with 7 recurrent genetic abnormalities.?[1] Conventional cytogenetics, interphase FISH, RT-PCR find application in the detection of recurrent genetic abnormalities as they play a pivotal role in prognostication, selection of therapy, and response to treatment.[1] [13] [31] [32] [33] [34] [35] [36] [37]
Translocation t(12;21)(p13;q22), a somatically acquired early lesion, results in the critical?TEL/AML1?fusion on chromosome 21, and?AML1/ TEL?fusion on derivative 12p that can be lost. TEL allele is often deleted the fusion protein acts in a dominant negative fashion inhibiting the normal function of transcription factor?AML1.[4] The abnormality can occur as a sole abnormality or within the context of a complex karyotype characterized by three or more chromosomal abnormalities. Conventional cytogenetics is the gold standard to detect both known and unknown genomic abnormalities in the genome; however, poor chromosome morphology, few malignant metaphases encountered in ALL, and inherent cytogenetic invisibility of the abnormality limit the detection of t(12;21) to 0.05% of patients. Cryptic and subtle abnormalities, below 7 to 8 MB resolution, can be missed on karyotyping. FISH and RT-PCR enable detection of?TEL/AML1?in 20 to 25% of precursor B ALL.[2] Translocation t(12;21) has a favorable outcome and useful for the prediction of outcome in childhood ALL. Coexistence of the same, with other gene rearrangements and in complex setting, favors poorer clinical outcome.
B-ALL patients with t(12;2l) abnormality are younger, ?3?5 years, blasts have B precursor phenotype, and are CD19, CD10 and CD34 positive with expression of myeloid antigens CD13, sensitive to chemotherapy and associated with favorable prognosis and late relapse.[1] [2] [3] [33]?TEL/AML1?fusion is seen rarely in adults (1?4%);[2] [3] however, increased numbers of adults diagnosed with B ALL, prompted assessment for the same. In the present study, under-representation of the cryptic?TEL/AML1?rearrangement overall?(8.4%)?and in children (13.6%) is low contrary to the expected high prevalence of 25% in western countries.[4] Hospital-based reports,[5] [6] [7] [9] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] international projects,[1] [10] [24] multicenter studies,[11] [24] and review articles[8] [<

|?Fig. 1TEL/AML1 positive case with an atypical signal pattern showing 1 fusion with additional copies of AML1 (green) and TEL (orange) gene in case 4 and an additional copy of only AML1 gene (green) in case 5 (clone 2).

|?Fig. 2Blasts showing high N/C ratio coarse chromatin and scanty cytoplasm (Leishman stain,400x).

|?Fig. 3Immunophenotyping in a prototype case showing aberrant expression of CD13 and CD33.
References
- JKC, Downing JR, Le Beau MM, Arber DA.?B lymphoblastic leukemia/lymphoma with recurrent genetic abnormalities. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. eds. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. 4th edition.. Lyon: IARC; 2017: 203-209
- aeim F, Rao PN.?Acute myeloid leukemia. In: Naeim F, Rao PN, Grody WW. eds. Hematopathology Morphology, Immunophenotype, Cytogenetics, and Molecular Approaches. 1st edition. Amsterdam: Elsevier; 2008: 207-255
- erwilliger T, Abdul-Hay M.?Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J 2017; 7 (06) e577
- elent A, Greaves M, Enver T.?Role of the TEL-AML1 fusion gene in the molecular pathogenesis of childhood acute lymphoblastic leukaemia. Oncogene 2004; 23 (24) 4275-4283
- hang L, Parkhurst JB, Kern WF. et al.?Chromosomal changes detected by fluorescence in situ hybridization in patients with acute lymphoblastic leukemia. Chin Med J (Engl) 2003; 116 (09) 1298-1303
- art?nez-Ram?rez A, Urioste M, Contra T. et al.?Fluorescence in situ hybridization study of TEL/AML1 fusion and other abnormalities involving TEL and AML1 genes. Correlation with cytogenetic findings and prognostic value in children with acute lymphocytic leukemia. Haematologica 2001; 86 (12) 1245-1253
- mid?ne A, Elghezal H, Sennana H. et al.?ETV6-RUNX1 rearrangement in Tunisian pediatric B-Lineage acute lymphoblastic leukemia. Adv Hematol. 2009;2009:924301.
- l-Sweedan SA, Neglia JP, Steiner ME, Bostrom BC, Casey T, Hirsch BA.?Characteristics of patients with TEL-AML1-positive acute lymphoblastic leukemia with single or multiple fusions. Pediatr Blood Cancer 2007; 48 (05) 510-514
- osad E, Hamed HB, Bakry RM, Ezz-Eldin AM, Khalifa NM.?Persistence of TEL-AML1 fusion gene as minimal residual disease has no additive prognostic value in CD 10 positive B-acute lymphoblastic leukemia: a FISH study. J Hematol Oncol 2008; 1: 17
- Siddiqui R, Nancy N, Naing WP. et al.?Distribution of common genetic subgroups in childhood acute lymphoblastic leukemia in four developing countries. Cancer Genet Cytogenet 2010; 200 (02) 149-153
- Iqbal Z.?Molecular genetic studies on 167 pediatric ALL patients from different areas of Pakistan confirm a low frequency of the favorable prognosis fusion oncogene TEL-AML1 (t 12; 21) in underdeveloped countries of the region. Asian Pac J Cancer Prev 2014; 15 (08) 3541-3546
- Khan A, Ayyub M, Ahmed S, Altaf CH, Malik HS.?Frequency of three different gene mutations (TEL-AML1, E2A?PBX1 and MLL-AF4) in acute lymphoblastic leukaemia. Hematol Transfus Int J 2016; 2: 00045
- Woo HY, Kim DW, Park H, Seong KW, Koo HH, Kim SH.?Molecular cytogenetic analysis of gene rearrangements in childhood acute lymphoblastic leukemia. J Korean Med Sci 2005; 20 (01) 36-41
- Chung HY, Kim KH, Jun KR. et al.?[Prognostic significance of TEL/AML1 rearrangement and its additional genetic changes in Korean childhood precursor B-acute lymphoblastic leukemia]. Korean J Lab Med 2010; 30 (01) 1-8
- Chen B, Wang YY, Shen Y. et al.?Newly diagnosed acute lymphoblastic leukemia in China (I): abnormal genetic patterns in 1346 childhood and adult cases and their comparison with the reports from Western countries. Leukemia 2012; 26 (07) 1608-1616
- Aydin C, Cetin Z, Manguoglu AE. et al.?Evaluation of ETV6/RUNX1fusion and additional abnormalities involving ETV6 and/or RUNX1genes using fish technique in patients with childhood acute lymphoblastic leukemia. Indian J Hematol Blood Transfus 2016; 32 (02) 154-161
- Zen PRG, Lima MC, Coser VM. et al.?Prevalence of TEL/AML1 fusion gene in Brazilian pediatric patients with acute lymphoblastic leukemia. Cancer Genet Cytogenet 2004; 151 (01) 68-72
- Yehuda-Gafni O, Cividalli G, Abrahmov A. et al.?Fluorescence in situ hybridization analysis of the cryptic t(12;21) (p13;q22) in childhood B-lineage acute lymphoblastic leukemia. Cancer Genet Cytogenet 2002; 132 (01) 61-64
- Sazawal S, Bhatia K, Gutierrez MI, Saxena R, Arya LS, Bhargava M.?Paucity of TEL-AML 1 translocation, by multiplex RT-PCR, in B-lineage acute lymphoblastic leukemia (ALL) in Indian patients. Am J Hematol 2004; 76 (01) 80-82
- Bhatia P, Binota J, Varma N. et al.?Incidence of common chimeric fusion transcripts in B-cell acute lymphoblastic leukemia: an Indian perspective. Acta Haematol 2012; 128 (01) 17-19
- Varma N, Naseem S, Binota J, Varma S, Malhotra P, Marwaha RK.?Study of incidence of recurrent genetic translocation in adult and pediatric acute lymphoblastic leukemia (ALL) patients in north India. International Journal of Epidemiology 2015;44:i234?35.
- Chopra A, Soni S, Verma D. et al.?Prevalence of common fusion transcripts in acute lymphoblastic leukemia: a report of 304 cases. Asia Pac J Clin Oncol 2015; 11 (04) 293-298
- Inamdar N, Kumar SA, Banavali SD, Advani S, Magrath I, Bhatia K.?Comparative incidence of the rearrangements of TEL/AML1 and ALL1 genes in pediatric precursor B acute lymphoblastic leukemias in India. Int J Oncol 1998; 13 (06) 1319-1322
- Siraj AK, Kamat S, Guti?rrez MI. et al.?Frequencies of the major subgroups of precursor B-cell acute lymphoblastic leukemia in Indian children differ from the West. Leukemia 2003; 17 (06) 1192-1193
- Pais AP, Amare Kadam PS, Raje GC. et al.?RUNX1 aberrations in ETV6/RUNX1-positive and ETV6/RUNX1-negative patients: its hemato-pathological and prognostic significance in a large cohort (619 cases) of ALL. Pediatr Hematol Oncol 2008; 25 (06) 582-597
- Kerketta LS, Baburao V, Ghosh K.?Pattern of chromosome involvement in childhood hyperdiploid pre-B-cell acute lymphoblastic leukemia cases from India. Indian J Hum Genet 2014; 20 (01) 32-36
- Magatha LS, Scott JX, Subramaniam G, Chandrasekaran T, Paul SFD, Koshy T.?Cytogenetic and fluorescence in Situ hybridization (FISH) profile of paediatric acute lymphoblastic leukemia (ALL) in a University Hospital in South India. Med Princ Pract 2021; 30: 563-570
- Mazloumi SH, Madhumathi DS, Appaji L, kumari Prasanna.?Combined study of cytogenetics and fluorescence in situ hybridization (FISH) analysis in childhood acute lymphoblastic leukemia (ALL) in a tertiary cancer centre in South India. Asian Pac J Cancer Prev 2012; 13 (08) 3825-3827
- Hill A, Short MA, Varghese C, Kusumakumary P, Kumari P, Morgan GJ.?The t(12:21) is underrepresented in childhood B-lineage acute lymphoblastic leukemia in Kerala, Southern India. Haematologica 2005; 90 (03) 414-416
- Siddaiahgari SR, Awaghad MA, Latha MS.?Clinical, immunophenotype and cytogenetic profile of acute lymphoblastic leukemia in children at tertiary health care centre in India. Muller J of Medical Sciences and Research 2015; 6: 112-118
- An International System for Human Cytogenomic Nomenclature (2016). Editors: McGowan-Jordan Simons A, Schmid M. Cytogenetic and Genome Research, Vol. 149, No.1?2, 2016.
- Marwaha RK, Kulkarni KP.?Childhood acute lymphoblastic leukemia: need of a national population based registry. Indian Pediatr 2011; 48 (10) 821
- Gujaral S, Badrinath Y, Kumar A. et al.?Immuno-phenotypic profile of acute leukemia: Critical analysis and insights gained at a tertiary care center in India. Blood 2008; 112: 4878
- Swaminathan R, Rama R, Shanta V.?Childhood cancers in Chennai, India, 1990-2001: incidence and survival. Int J Cancer 2008; 122 (11) 2607-2611
- Arora RS, Eden TO, Kapoor G.?Epidemiology of childhood cancer in India. Indian J Cancer 2009; 46 (04) 264-273
- Tyagi BB, Manoharan N, Raina V.?Childhood cancer incidence in Delhi, 1996?2000. Indian J Med Paediatr Oncol 2006; 27: 13-18
- Ma SK, Wan TSK, Cheuk ATC. et al.?Characterization of additional genetic events in childhood acute lymphoblastic leukemia with TEL/AML1 gene fusion: a molecular cytogenetics study. Leukemia 2001; 15 (09) 1442-1447
- Yang M, Yi ES, Kim HJ, Yoo KH, Koo HH, Kim SH.?Intrachromosomal amplification of chromosome 21 in Korean pediatric patients with B-cell precursor acute lymphoblastic leukemia in a single institution. Blood Res 2017; 52 (02) 100-105
- Harrison CJ, Haas O, Harbott J. et al; Biology and Diagnosis Committee of International Berlin-Frankf?rt-M?nster study group.?Detection of prognostically relevant genetic abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: recommendations from the Biology and Diagnosis Committee of the International Berlin-Frankf?rt-M?nster study group. Br J Haematol 2010; 151 (02) 132-142


 PDF
PDF  Views
Views  Share
Share

