Report of chronic myeloid leukemia in chronic phase from Cancer Institute (Women India Association), Chennai, 2002-2009
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 206-207
DOI: DOI: 10.4103/0971-5851.123744
Abstract
Cancer Institute Chennai is the first Institute of Oncological sciences to be established in the country. In ICON meeting, they presented the data of 516 patients, of which 91% patients achieved complete hematological response. The overall survival was 88% and event free survival was 65% at 5 years.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Cancer Institute Chennai is the first Institute of Oncological sciences to be established in the country. In ICON meeting, they presented the data of 516 patients, of which 91% patients achieved complete hematological response. The overall survival was 88% and event free survival was 65% at 5 years.
INTRODUCTION
Imatinib came in vogue since 2000, as being convenient, effective and a drug with tolerable side-effects.[1,2] In our patients population, treated over a period of 7 years, we concluded that around 10% of patient population had hematological toxicity, while 15-20% had non-hematological toxicity. The overall survival OAS was 85%, which is comparable to any published literature,[3] indicating the Imatinib is effective drug and can be easily tolerated without much side effects.
PATIENT AND METHODS
It was retrospective analysis of newly diagnosed chronic myeloid leukemia patients registered at our institute from year 2002 to 2009.
RESULTS
Presenting features
The total number of evaluable patients in this period is 516 and there were 343 males and 172 females; male: Female ratio: 2:1. The median age at presentation was 35 years. The mean duration of symptoms at presentation was 3.6 months. Most of them (77.9%) had received prior hydroxyl urea though mostly for less than 1 month. Nearly 2% had received busulfan and 1.4% had received interferon. 515/516 patients had splenomegaly. The median hemoglobin was 10 g/dl, the white cell count was 1.86 lakhs/cumm and the platelet count was 3.47 lakhs/cumm. The Sokal risk score distribution of the cases was as follows: Low-25%, intermediate 47%, high-28%.
Treatment and response
All were started on imatinib 400 mg/day. 470 (91.1%) achieved complete hematological response (CHR). Among those who achieved CHR (n = 470), 347 (73%) achieved it within 3 months while 123 (27%) took more than 3 months to achieve CHR. Among those in which cytogenetic assessment was done at some point, 79% achieved major cytogenetic response and 40% achieved complete cytogenetic response. During the follow-up, 66 patients out of the 470 who achieved CHR, lost CHR (14%). The median follow-up for the entire cohort was 38.9 ± 22 months (range: 1-96 months). The median event free survival (EFS) was 79.3 months (5 year EFS 65%). The overall survival was 88.5% at 5 years (median was not reached). Figure 1 shows the graph of EFS.
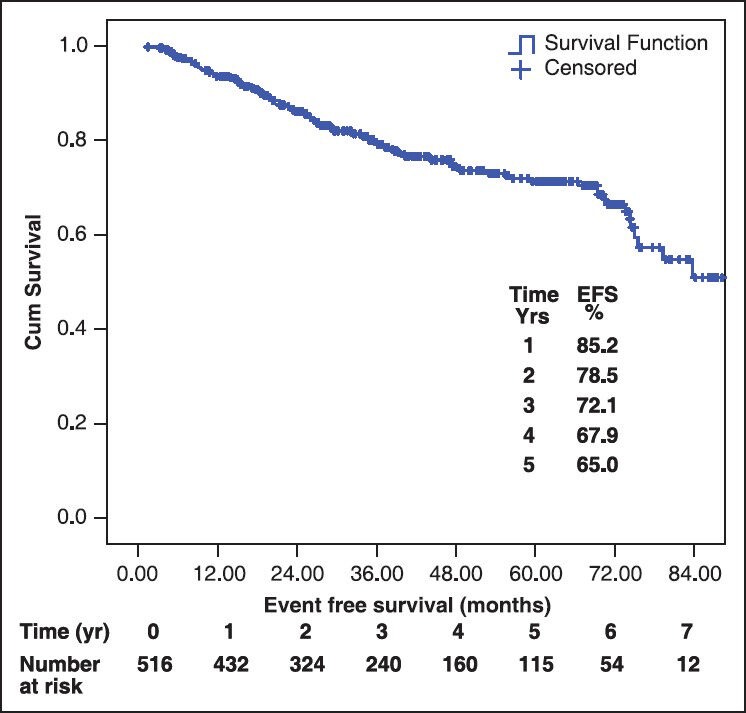
| Fig. 1 Event free survival of chronic myeloid leukemia patients
Dose escalation was carried out in 148 patients; of these 36% had response, which was suboptimal, 25% had an optimal response, 35% there was no effect/progression and 2.3% couldn’t tolerate the higher dose.
Toxicity assessment
The most common non-hematological toxicity was related to skin depigmentation, which was seen in 92 patients (15.6%). The other common toxicities were edema (8%), transaminitis (0.7%), skin rashes in 2%, cramps and myalgias in 16%. Most of the non-hematological toxicities were grade ½. Grade ¾ hematological toxicity was seen in 11% patients (anemia 2%, thrombocytopenia 5.5% and neutropenia 11%).
DISCUSSION
In our cohort of patients, 91% patients achieved CHR, out of which 73% were able to achieve it in within 3 months. Dose escalation was required in 28% of patients, out of which 36% responded while 35% progressed or had no response. This data of ours is comparable to already published literature and emphasize that CML patients needs to be followed up regularly to improve the overall outcome.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Event free survival of chronic myeloid leukemia patients


 PDF
PDF  Views
Views  Share
Share

