Report of chronic myeloid leukemia from Indira Gandhi Institute of Medical Sciences, Regional Cancer Center, 2002-2009
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 172-174
DOI: DOI: 10.4103/0971-5851.123719
Abstract
Indira Gandhi Institute of Medical Sciences, Regional Cancer Center was established in 1993. It′s one of the main Health-Care Institution in the state of Bihar. The data of 205 patients was presented in the ICON meeting and 98% of patients were diagnosed in chronic phase. Complete hematological response was seen in 91% of patients in 3 months. A total of 197 (96%) patients were alive at the time of analysis of which 179 (87%) were still in chronic phase with hematological remission.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Indira Gandhi Institute of Medical Sciences, Regional Cancer Center was established in 1993. It's one of the main Health-Care Institution in the state of Bihar. The data of 205 patients was presented in the ICON meeting and 98% of patients were diagnosed in chronic phase. Complete hematological response was seen in 91% of patients in 3 months. A total of 197 (96%) patients were alive at the time of analysis of which 179 (87%) were still in chronic phase with hematological remission.
INTRODUCTION
Chronic myeloid leukemia (CML) is the most common leukemia seen at Regional Cancer center, Indira Gandhi Institute of Medical Sciences, Sheikhpura, Patna and Bihar, India. CML cases constitute about 70% of total leukemia cases seen at our institution, whereas in India, CML accounts for 30% to 60% of all adult leukemia.[1,2] In India, its incidence varies from 0.8 to 2.2 per hundred thousand population in males and 0.6-1.6/1 lakh population in females.[1] The introduction of Imatinib mysalate in 2002 has virtually changed the prognosis of patients with CML.[3] Majority of our patients were from the various districts of Bihar, with a large number of cases coming from districts of Samastipur, Patna and Dharbhanga. Furthermore, many of these patients came from neighboring states such as Jharkhand, Uttar Pradesh, especially from the eastern part, Delhi, West-Bengal, Madhya Pradesh, Orissa and Chhattisgarh.
Approximately, 85% of patients were from a low to middle socio-economic strata and hence were unable to afford Imatinib. They extremely benefited from Glivec International Patient Assistance Program as our center is the only approved center in the entire state of Bihar. We are thankful to Max foundation and Novartis Oncology Access Program for their wholehearted support to the poor and needy patients. Here, we report our experience of patients of CML undergoing treatment in limited resource set-up.
PATIENTS AND METHODS
This is a retrospective analysis and the data was extracted from clinical case files of patients registered at Indira Gandhi Institute of Medical Sciences since January 2002 to December 2009. All patients were Philadelphia chromosome positive (examined by fluorescence in situ hybridization method on bone marrow sample) and were treated with Imatinib mesylate. The aims and objective of this retrospective analysis were to document the clinical profile of patients of CML patients, response and compliance to Imatinib and to evaluate its toxicity profile.
RESULTS
The total number of patients registered and treated with Imatinib mesylate during this period was 205, out of which females were 54 (26%) versus males who were 151 (74%). It was observed that 6 (3%) of patients were less than 20 years of age; whereas 135 (66%) were between 20 and 40 and 64 (31%) were above 40 years of age. The baseline clinical characteristics are shown in Table 1.
Table 1
Clinical characteristics
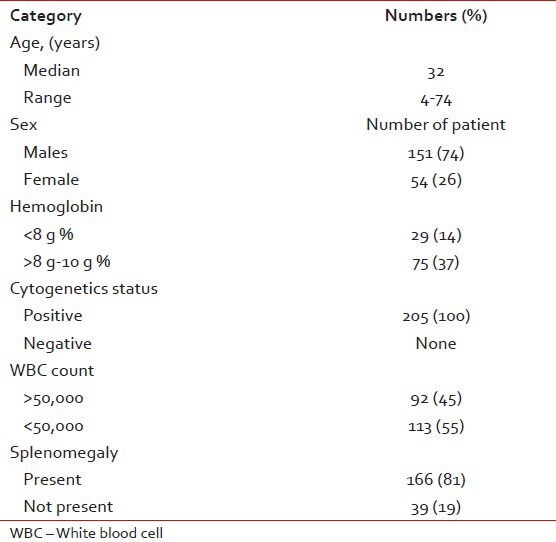
Majority presented to us in chronic phase except 5 (2.4%) who presented initially in blast crises/accelerated phase. Total of 189 (92%) of patients had fatigue and weakness at the time of diagnosis. Splenomegaly a leading manifestation was associated in 166 (81%) of patients, whereas lymphadenopathy was present in 4 (2%). At the time of diagnosis 84 (41%) patients had fever. And 92 (45%) of patients had total leukocyte count more than 50,000. Anemia was present in 75 (37%) of patients and in 29 (14%) cases had less than 8 g/dl of hemoglobin. They required blood transfusion at the time of diagnosis. No patient required platelets support. Further 97 (47%) patients required initial treatment with hydroxyurea. The median time from diagnosis to start of Imatinib therapy was 2.6 months (ranged from 24 days to 4 years) in our subset of patients. Patients were monitored on a 3 monthly basis with hemogram, liver and kidney profile, a complete physical examination as far as possible.
Response to treatment
Hematological response was seen in 187 (91%) of patients at 3 months. The response to treatment is shown in Table 2. Analysis for BCR-ABL was repeated after 6th month – 2 years depending upon affordability of the patients. Cytogenetic response was assessed in 127 (62%) of patients at one point of time after initiating therapy. All of whom had complete/major cytogenetic response. Two of our female patients had associated pregnancy. One of them while on treatment for CML underwent a medical termination of pregnancy; while the other was diagnosed with CML during the 7th month of pregnancy and Imatinib was started only after the uneventful delivery of a full-term normal baby boy. It was possible to manage the case without drugs during pregnancy. She has been on follow-up and is in complete molecular remission at the end of 1 year of treatment.
Table 2
Responses to treatment

Common adverse events
117 (57%) patient presented with non-hematological toxicities in the form of hypopigmentation 87 (42%), Rashes 49 (12%). Pigmentation over malar area was seen in the majority of cases while on imatinib therapy. Fluid retention 13 (6%), weight gain 35 (17%), musculoskeletal pain was also associated in 31 (15%) as shown in Table 3.
Table 3
Common adverse event to imatinib mesylate
Hematological toxicity in the form of anemia was seen in 13 (6%), which required blood transfusion during treatment. Thrombocytopenia was seen in 2 (4%). Liver toxicity was associated in the form of deranged enzyme levels in 17 (8%), which however did not require treatment interruption. Ototoxicity was surprisingly seen in 5 (2.4%) patients, one whom presented just within 3 days of start of Imatinib. Decrease in vision occurred in 2 (1%) patients. Two of our patients developed Chloroma and were treated with radiotherapy.
A total of 197 (96%) patients were alive at the time of analysis of which 179 (87%) were still in chronic phase with hematological remission. About 6 patients were in accelerated phase and were being treated with higher dose of Imatinib. The actual status of 12 patients was unknown at the time of analysis. Over all 182 (89%) of patients said they had a better quality-of-life with treatment.
DISCUSSION
Bihar is a poor state with the per capita income being one of the lowest in the country. It has been shown in a number of studies that CML tends to afflict people at a younger age in Asia than in western nations. Instead of being diagnosed in their 40s and 50s, patients in India are more likely to be in their 20s and 30s. Our analysis confirmed that the relative age of patient presenting to us was younger than western population.[4] The male: Female ratio was 3:1, which is more than that documented in the literature suggesting a gender bias.[1] Majority of the patients presented in chronic phase of the disease. Rashes and hypo pigmentation was more in our patients sub population as related to western data. Dose of Imatinib was increased due to poor response in the minority of patients. Monitoring responses was difficult because of outstation visits and financial implications and despite these constrains majority of the patients have done well. The hematological and cytogenetic response was similar to reported in other Indian studies.[5] many of the patients who were largely asymptomatic had difficulty in accepting that they have a potentially lethal disease. Some patients also explored Ayurvedic or other alternative treatments.
Single institution data is unlikely to give a complete picture indicative of all Indian patients. It is proposed to have collation of data from different institutions in Indian treating CML in India to have a larger picture of CML patients in India.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

