Rational Use of Imaging to Stage Breast Cancer: Evidences for a Selective Approach
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 427-429
DOI: DOI: 10.4103/ijmpo.ijmpo_113_16
Abstract
Introduction: Staging investigations at diagnosis are customary to accurately assign a clinical stage before therapy. The practice of routine imaging in patients asymptomatic for metastasis is not recommended but widely adopted. This study was done to reexamine the basis behind guideline recommendations and to identify the factors predictive of asymptomatic metastasis. Methods: Oncology records of 200 breast cancer patients in clinical Stages I-III at diagnosis were prospectively reviewed. Baseline demographic information, tumor characteristics, and pathological data including molecular typing were collected. The prevalence of metastasis deduced and accuracy of bone scan, chest X-ray (CXR), liver ultrasound, andcomputed tomography (CT) chest analyzed. Patient and tumor characteristics predictive of asymptomatic metastasis tested for significance using appropriate statistical tests. Results: The prevalence of asymptomatic metastasis was 13.5%. Bone lesions (8%) were the mostcommon metastatic site followed by lungs (7%) and liver (1%). Sensitivity, specificity, positive- and negative-predictive values of bone scans and CT chest were 100%, 97%, 74%, 100%, and 92%, 99%, 87, 3%, 99.4%, respectively. The above values for ultrasound abdomen and CXRs were 100%, 99%, 93%, 100% and 21%, 94%, 20%, 94%, respectively. Tumor size (P = 0.001), tumor Stage T1/T2 versus T3/T4 (P = 0.0002), nodal stages N0/N1 versus N2/N3 (P = 0.001), high histological Grade G I versus GII/GIII (P = 0.0001) and molecular types were strongly predictive of metastatic disease. Conclusion: The routine use of imaging to detect distant metastasis in asymptomatic patients is not recommended in newly diagnosed breast cancer. A selective approach may be adopted in individuals with tumor more than 5 cm, advanced nodal disease, higher histological grade, and aggressive molecular types.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Staging investigations at diagnosis are customary to accurately assign a clinical stage before therapy. The practice of routine imaging in patients asymptomatic for metastasis is not recommended but widely adopted. This study was done to reexamine the basis behind guideline recommendations and to identify the factors predictive of asymptomatic metastasis.
Methods:
Oncology records of 200 breast cancer patients in clinical Stages I-III at diagnosis were prospectively reviewed. Baseline demographic information, tumor characteristics, and pathological data including molecular typing were collected. The prevalence of metastasis deduced and accuracy of bone scan, chest X-ray (CXR), liver ultrasound, and computed tomography (CT) chest analyzed. Patient and tumor characteristics predictive of asymptomatic metastasis tested for significance using appropriate statistical tests.
Results:
The prevalence of asymptomatic metastasis was 13.5%. Bone lesions (8%) were the most common metastatic site followed by lungs (7%) and liver (1%). Sensitivity, specificity, positive- and negative-predictive values of bone scans and CT chest were 100%, 97%, 74%, 100%, and 92%, 99%, 87, 3%, 99.4%, respectively. The above values for ultrasound abdomen and CXRs were 100%, 99%, 93%, 100% and 21%, 94%, 20%, 94%, respectively. Tumor size (P = 0.001), tumor Stage T1/T2 versus T3/T4 (P = 0.0002), nodal stages N0/N1 versus N2/N3 (P = 0.001), high histological Grade G I versus GII/GIII (P = 0.0001) and molecular types were strongly predictive of metastatic disease.
Conclusion:
The routine use of imaging to detect distant metastasis in asymptomatic patients is not recommended in newly diagnosed breast cancer. A selective approach may be adopted in individuals with tumor more than 5 cm, advanced nodal disease, higher histological grade, and aggressive molecular types.
Introduction
Breast cancer is the most common female malignancy and most women present with early stage disease. Conventionally, routine pretherapy noninvasive investigations including radiological imaging is done to assign a clinical disease stage at diagnosis. Accurate stage determination of breast cancer at presentation is essential to decide on primary loco regional therapy versus systemic therapy. The application of these staging tests in early breast cancer has been questioned as the detection of metastatic disease is low.[1] Several practice guidelines have suggested against the routine use of staging imaging in asymptomatic patients.[2,3] However, despite recommendations reports confirm the continual practice of pursuing routine staging investigations in newly diagnosed breast cancer. Inappropriate imaging in early stage breast cancer may increase treatment expenses and potentially lead to harm by false positive results.[4]
It is difficult to understand the reluctance among oncologist to follow practice guideline recommendations regarding staging investigations in breast cancer. The accuracy of a diagnostic test depends on the pretest probability of detecting disease consequently applying staging investigations to high-risk groups is likely to enhance identification of asymptomatic metastasis.[5] In this study, we sought to reexamine the basis behind the guideline practice recommendations and to recognize predictive factors that could identify disease at high risk of asymptomatic metastasis.
Methods
The oncology records of newly diagnosed breast cancer patients attending our center were reviewed for study inclusion. Patients presenting with symptomatic metastatic breast cancer were excluded from the study. Data from 200 patients in clinical Stages I-III (AJCC Stage 7th edition) and asymptomatic for metastatic disease were retrieved and included in the study. Demographic information, tumor characteristics, and pathological data were collected, molecular typing followed the St. Gallen 2011 recommendations. Our center follows a policy of liver ultra sound examination and chest X-ray (CXR) in addition to routine hematological and liver biochemistry studies in early breast cancer. Isotope bone scan, computed tomography (CT) chest and ultra sound/CT abdomen are done as staging imaging in clinical Stage III breast cancer. The radiological reports of bone scan, CT chest, CXR, and abdominal ultrasound of study patients were examined and included for analysis. The overall prevalence rate of asymptomatic metastatic disease deduced and applied for the positive- and negative-predictive value calculations (PPV and NPV). The study applied a definition of unequivocal radiological report of metastatic disease (multiple) as a true positive result. Solitary or asymmetric tracer uptake in bone scans were confirmed by magnetic resonance imaging/CT scans to confirm metastatic disease. Solitary lesions in liver ultrasound were confirmed by CT abdomen or a biopsy, a similar policy was followed for solitary pulmonary/indeterminate lung lesions. Patients with normal staging imaging results but developed symptomatic metastasis while under therapy were deemed to have had a false negative imaging study.
The prevalence of asymptomatic metastatic disease, sensitivity, specificity, and predictive values of staging imaging investigations were calculated. An analysis of tumor and patient related factors to identify predictive factors for metastatic disease was done. Categorical variables were analyzed using Fisher's exact test or Chi-square test and ordinal data tested for significance using the Student's t-test. A P < 0>
Results
The mean age of the study patients was 50 years, 27 (13.5%) symptomatic metastasis was identified, and this formed the prevalence value for subsequent analysis. Bone lesions (8%) were the most common metastatic site followed by lungs (7%) and liver (1%). Ten patients (37%) had a single system involvement, while axial skeleton and the lungs were the most common combination noted. Sensitivity, specificity, positive- and negative-predictive values (PPV and NPV) of bone scans and CT chest were 100%, 97%, 74%, 100% and 92%, 99%, 87, 3%, 99.4%, respectively. The above values for ultrasound abdomen and CXRs were 100%, 99%, 93%, 100% and 21%, 94%, 20%, 94%, respectively [Table 1]. An analysis of factors predictive of metastatic disease was done and showed age (P = 0.24) and menopausal status (P = 1) had no influence on detection of metastasis. Tumor size (P = 0.001), tumor stage T1/T2 versus T3/T4 (P = 0.0002), nodal stages N0/N1 versus N2/N3 (P = 0.001), and high histological Grade G I versus GII/GIII (P = 0.0001) were strongly predictive of metastatic disease. Among the molecular sub types of breast cancer the luminal A was the least aggressive P = 0.003, but the human epidermal growth factor receptor 2 (HER 2) (P = 0.04) and triple negative (P = 0.02) types significantly predicted higher metastatic disease [Table 2].
Table 1
Diagnostic accuracy of staging radiological imaging
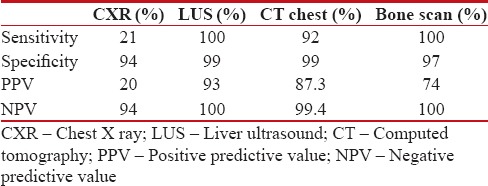
|
Table 2
Factors predictive of asymptomatic metastasis
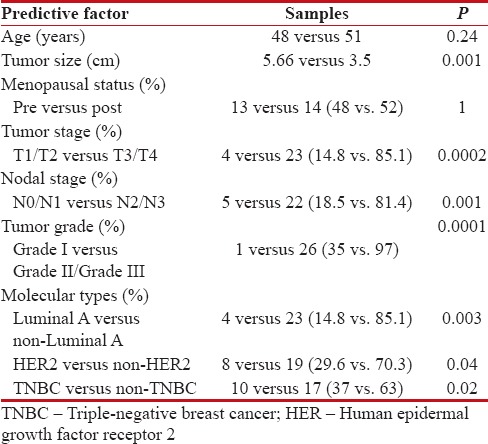
|
Discussion
Our study detected a metastatic prevalence of 13.6% in clinical Stage I-III breast cancer. The prevalence of distant disease in breast cancer patients asymptomatic for metastasis varies in literature from 2% to 10%.[1,6,7] Greater proportion of patients presenting in Stage III disease in this study compared to other published series could explain the high prevalence of metastatic disease observed, as Indian women typically present late and metastatic disease show a linear increase with advancing tumor stage.
The incidence of bone metastasis in breast cancer is variable. Radioisotope bone scans are sensitive than skeletal survey in detecting bone metastasis. The reported sensitivity of isotope bone scans varies and false positive rates are high as benign processes are also detected. Asymptomatic bone metastasis was detected in 8% of the study patients with a sensitivity, specificity, PPV, and NPV of 100%, 97%, 74%, and 100%, respectively. The prevalence of bone lesions and accuracy of bone scan in detecting metastasis matches with the results of Puglisi et al.[6] An evidence-based review that included 20 studies reports a sensitivity of 98% but cautions a high false positive rate of 10%–22% and a false negative rate of 10%. The review also noted a declining trend in positive bone scans from 10% to 3%-between studies published earlier and after 1985.[2] In addition, our results also confirm a linear association of bone metastasis with increasing clinical stage.
Liver ultrasound has been recommended to screen for liver metastasis in breast cancer. Earlier studies have shown a yield of 0%, 0.45%, and 2% for Stage I, II, and III disease.[2] Overall, our study detected hepatic metastasis in 2%-with 0%, 2%, and 4%-detected in Stage I, II, and III disease. While some authors have argued against routine liver ultrasound examination, a recent report suggests a molecular typing based selection approach to metastatic work up.[2,6,7] This report found a significant association of HER 2 type with liver metastasis.[8] The marginal higher prevalence for liver metastasis noted in this study compared to contemporary reports of Chen et al. remains unexplainable.
CXR detected a metastasis is <1 xss=removed>et al. which reported only a yield of 0.099%.[9] A review noted a yield of only, 0.1%, 0.2%, and 1.7% in Stage I, II, and III breast cancer.[2] Our study also confirms the low sensitivity (21%) and PPV (20%) of CXRs to detect metastatic disease. CT chest as a single staging modality replacing bone scans and ultrasound abdomen has been suggested. CT chest in our study demonstrated a sensitivity of 92%-this finding is concordant with Barrett et al.[10] It may be noted that accuracy indices of the staging imaging are variable than that observed in literature, however, these indices are known to be influenced by study definitions and the pretest probability (prevalence) of detecting disease in the study cohort. A higher prevalence in our study may have influenced higher accuracy indices noted.
An analysis of predictive factors for detecting metastatic disease revealed a significant association with tumor size more than 5 cm, N2/N3 nodal status and higher histological grade (Grade II and III). A similar finding has been reported in literature.[10,11] The luminal A subtype was the least associated with metastasis and the HER 2 positive and triple negative types associated with high prevalence of asymptomatic metastasis at diagnosis. No difference in the risk of symptomatic metastasis was noted between luminal A and B type. These observations are consistent with the report by Chen et al.[8] Our results are analogous with the ESMO breast cancer guideline which recommends a chest CT scan, ultrasound or CT scan of the abdomen and bone scan in biologically aggressive tumors, tumors more than 5 cm in size, clinically significant axillary nodal disease and abnormal biochemistry suggestive of metastasis.
Conclusion
Although our study detected a higher prevalence of asymptomatic metastatic disease in breast cancer, the yield of routine staging investigations is minimal. The routine use of imaging to detect distant metastasis in asymptomatic patients is not recommended in newly diagnosed breast cancer. An individualized approach may be adopted in individuals with tumor more than 5 cm, advanced nodal disease, higher histological grade, and aggressive molecular types.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Myers RE, Johnston M, Pritchard K, Levine M, Oliver T, Breast Cancer Disease Site Group of the Cancer Care Ontario Practice Guidelines Initiative, et al. Baseline staging tests in primary breast cancer: A practice guideline. CMAJ 2001;164:1439-44.
- Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13866. [Last accessed on 2015 Nov 26].
- Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8-30.
- Simos D, Catley C, van Walraven C, Arnaout A, Booth CM, McInnes M, et al. Imaging for distant metastases in women with early-stage breast cancer: A population-based cohort study. CMAJ 2015;187:E387-97.
- Watson RA, Tang DB. The predictive value of prostatic acid phosphatase as a screening test for prostatic cancer. N Engl J Med 1980;303:497-9.
- Puglisi F, Follador A, Minisini AM, Cardellino GG, Russo S, Andreetta C, et al. Baseline staging tests after a new diagnosis of breast cancer: Further evidence of their limited indications. Ann Oncol 2005;16:263-6.
- Samant R, Ganguly P. Staging investigations in patients with breast cancer: The role of bone scans and liver imaging. Arch Surg 1999;134:551-3.
- Chen X, Sun L, Cong Y, Zhang T, Lin Q, Meng Q, et al. Baseline staging tests based on molecular subtype is necessary for newly diagnosed breast cancer. J Exp Clin Cancer Res 2014;33:28.
- Chen EA, Carlson GA, Coughlin BF, Reed WP Jr., Garb JL, Frank JL, et al. Routine chest roentgenography is unnecessary in the work-up of stage I and II breast cancer. J Clin Oncol 2000;18:3503-6.
- Barrett T, Bowden DJ, Greenberg DC, Brown CH, Wishart GC, Britton PD, et al. Radiological staging in breast cancer: Which asymptomatic patients to image and how. Br J Cancer 2009;101:1522-8.
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181-7.
References
- Myers RE, Johnston M, Pritchard K, Levine M, Oliver T, Breast Cancer Disease Site Group of the Cancer Care Ontario Practice Guidelines Initiative, et al. Baseline staging tests in primary breast cancer: A practice guideline. CMAJ 2001;164:1439-44.
- Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13866. [Last accessed on 2015 Nov 26].
- Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8-30.
- Simos D, Catley C, van Walraven C, Arnaout A, Booth CM, McInnes M, et al. Imaging for distant metastases in women with early-stage breast cancer: A population-based cohort study. CMAJ 2015;187:E387-97.
- Watson RA, Tang DB. The predictive value of prostatic acid phosphatase as a screening test for prostatic cancer. N Engl J Med 1980;303:497-9.
- Puglisi F, Follador A, Minisini AM, Cardellino GG, Russo S, Andreetta C, et al. Baseline staging tests after a new diagnosis of breast cancer: Further evidence of their limited indications. Ann Oncol 2005;16:263-6.
- Samant R, Ganguly P. Staging investigations in patients with breast cancer: The role of bone scans and liver imaging. Arch Surg 1999;134:551-3.
- Chen X, Sun L, Cong Y, Zhang T, Lin Q, Meng Q, et al. Baseline staging tests based on molecular subtype is necessary for newly diagnosed breast cancer. J Exp Clin Cancer Res 2014;33:28.
- Chen EA, Carlson GA, Coughlin BF, Reed WP Jr., Garb JL, Frank JL, et al. Routine chest roentgenography is unnecessary in the work-up of stage I and II breast cancer. J Clin Oncol 2000;18:3503-6.
- Barrett T, Bowden DJ, Greenberg DC, Brown CH, Wishart GC, Britton PD, et al. Radiological staging in breast cancer: Which asymptomatic patients to image and how. Br J Cancer 2009;101:1522-8.
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181-7.


 PDF
PDF  Views
Views  Share
Share

