R248W Mutations in p53 Gene are Rare Among Indian Patients with Head-and-Neck Cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(04): 519-522
DOI: DOI: 10.4103/ijmpo.ijmpo_248_19
Abstract
Aim
Cancer is one of the curses to humankind, decades of research in eradicating the disease from the society is proven difficult. Close interaction between clinicians and scientists helps us to translate clinical observations into molecular mechanism of the disease. The Cancer Genome Atlas data suggest that genetic alterations in p53 gene play a crucial role in head-and-neck squamous cell carcinoma (HNSCC) tumorigenesis. Understanding p53 aberrations and their impact on other cellular activities can help with the design of new, more effective therapeutic strategy that target p53 mutation-bearing HNSCC, thereby producing a personalized medicine approach for the disease.
Materials and Methods
In an effort to identify the role of R248W mutation of p53 gene in HNSCC patients of Indian origin, tumor samples were collected from 55 patients (n = 55), and polymerase chain reaction–restriction fragment length polymorphism technique was used to screen for the mutation using genomic DNA isolated from the tumors.
Results
The results reveal that except for one patient (heterozygous), all the patients were negative for the mutation.
Conclusion
These results suggest that p53 R248W mutations are less prevalent in HNSCC Indian patients.
Keywords
Head-and-neck cancer - mutation - p53 - polymerase chain reaction–restriction fragment length polymorphismPublication History
Received: 13 December 2019
Accepted: 29 June 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aim
Cancer is one of the curses to humankind, decades of research in eradicating the disease from the society is proven difficult. Close interaction between clinicians and scientists helps us to translate clinical observations into molecular mechanism of the disease. The Cancer Genome Atlas data suggest that genetic alterations in p53 gene play a crucial role in head-and-neck squamous cell carcinoma (HNSCC) tumorigenesis. Understanding p53 aberrations and their impact on other cellular activities can help with the design of new, more effective therapeutic strategy that target p53 mutation-bearing HNSCC, thereby producing a personalized medicine approach for the disease.
Materials and Methods
In an effort to identify the role of R248W mutation of p53 gene in HNSCC patients of Indian origin, tumor samples were collected from 55 patients (n = 55), and polymerase chain reaction–restriction fragment length polymorphism technique was used to screen for the mutation using genomic DNA isolated from the tumors.
Results
The results reveal that except for one patient (heterozygous), all the patients were negative for the mutation.
Conclusion
These results suggest that p53 R248W mutations are less prevalent in HNSCC Indian patients.
Keywords
Head-and-neck cancer - mutation - p53 - polymerase chain reaction–restriction fragment length polymorphismIntroduction
Head-and-neck squamous cell carcinoma (HNSCC), a major form of head-and-neck cancer, is an important clinical challenge in oncology and is the sixth most common cancer in the world today.[1] More than 600,000 new head and neck cancer cases are diagnosed annually in the world, with about 350,000 deaths.[2] The Cancer Genome Atlas data suggest that genetic alterations in p53 gene play a crucial role in HNSCC tumorigenesis.[3] Identification of various gene mutations in cancer patients and relating their impact on cancer progression and recurrence will give us an insight into the role played by these mutations in cancer.
The p53 gene product is involved in regulating several key events in the cell that are essential for cell growth and suppression of malignancy. Thus, mutation in p53 induces malignancy.[4] When DNA gets damaged, wild-type p53 protein accumulates and stops replication to repair the DNA. This is followed by triggering specific cell cycle arrest in the G1/S phase. In case of tumor cells bearing mutated p53, this cell cycle arrest is not induced, which corresponds to an increase in various somatic mutations in cells that help the progression of cancer.[5] Inactivation of tumor suppressor gene p53 plays a key role in cancer development. The p53 gene is frequently lost or mutated in several kinds of tumors, including the colon, lung, breast, brain, ovary, and esophagus implicating p53 as an important tumor suppressor gene.[6] In head-and-neck cancer, alterations of p53 gene have been implicated at a high incidence of the disease.[7]
In several forms of cancer, p53 is mutated and codon 248 is one of the hot spot regions which represents 60%-of known p53 mutations.[8] In HNSCC also, 75%- to 85%-of human papillomavirus-negative patients possess mutations in TP53 and most of the mutations are frequently observed within the DNA binding domain of the p53 protein.[9] All p53 mutations described in HNSCC occur in highly conserved regions between exon 5 and 8.[10] In head-and-neck cancer, p53 mutations are significantly associated with short survival rate and often resistant to standard treatments including radiotherapy and chemotherapy. This indicates that for head-and-neck cancer patients, p53 mutations can be used as a prognostic biomarker. In p53 protein, since codon 248 is one of the hot spot mutations observed in several forms of cancer and there is no report available on the prevalence of this mutation in Indian HNSCC patients, this study was carried out.
Materials and Methods
Patients and specimens
Tumor samples used in this study were surgically excised from 55 head-and-neck cancer patients at Apollo hospital, Chennai, India. After resection, the tumors were snap-frozen and then transported to VIT University, Vellore. All patients were followed up and the data concerning cancer recurrence and patient survival were collected. We obtained informed consent from all the individuals who participated in the study as well as approval from the institute's human ethical committee (IEC/IRB No: IECH/2013/Dec18-006). All patients were Indian residents from the states of Tamil Nadu, West Bengal, Andhra Pradesh, Manipur, and Pondicherry.
DNA extraction
DNA from 55 tumor samples was extracted by a high salt method. The tumor tissue was processed by grinding the fresh sample using a motor and pestle in liquid nitrogen. For protease digestion, the tissue was transferred into a microfuge tube containing 1 ml of Tris Nacl EDTA SDS (10 mM Tris, 6M NaCl, 100 mM ethylenediaminetetraacetic acid, and 0.6%-sodium dodecyl sulfate) buffer with 60 μl of proteinase-k (20 mg/ml) and the tubes were incubated overnight at 45°C. At the end of the incubation period, 277 μl of 6M NaCl was added, mixed, and centrifuged for 12,000 RPM for 10 min. The supernatant was transferred to a fresh microfuge tube, and DNA was precipitated by adding equal volume of 100%-ethanol. The DNA was pelleted by centrifugation at 12,000 RPM for 10 min. After centrifugation, the supernatant was discarded and the pellet was washed with 70%-ethanol and air-dried. The recovered DNA was suspended in 20–100 μl of sterile distilled water. The DNA was quantitated by using NanoDrop instrument (Thermo Fisher scientific) and quality of the DNA was tested on agarose gel electrophoresis.
Polymerase chain reaction–restriction fragment length polymorphism
Polymerase chain reaction (PCR) amplification of p53 gene at codon 248 was performed in 50 μl of reaction mixture containing 5 μl of 10x buffer, 2 μl of each primer, 4 μl of dNTP mixture (2.5 mM), 0.4 μl of Taq polymerase (5units/ml) (Takara, Japan), 1 μl of template DNA (50 ng/μL), and 35.6 μl of H2O. The profile used in the PCR (Eppendorf Master cycler Nexus cycler) was 30 s at 94°C, 45 s at 68°C, 72°C for 50 s for 35 cycles, and 5 min at 72°C. The PCR product was digested with Msp1 restriction enzyme (New England Biolabs) and the digested products were visualized under ultraviolet light on the gel documentation system (Syngene) after electrophoresis on a 2%-agarose gel containing ethidium bromide. The primers used was Forward: 5'TGG TGC TGG GCA CCT GTA GTC CCA GCT ACT CG3' and Reverse: 5' ACT ACT CAG GAT AGG AAA AGA GAA GCA AGA GGC3'.[11]
Results
p53 mutation using polymerase chain reaction–restriction fragment length polymorphism
p53 mutation detection was assessed for 55 samples using PCR-restriction fragment length polymorphism (PCR-RFLP) method. For determining the mutations in the codon 248, PCR-amplified fragments of p53 gene (680 base pair) were digested with restriction enzyme Msp1 for 1 h at 37°C and electrophoresed in 2%- agarose gel containing ethidium bromide. Upon restriction, the wild type resulted in 170, 220, and 290 base pair product for the CGG (R/R) in codon 248. Samples with 170, 220, 290, and 460 base pairs represent the heterozygous mutation (HTM) since the CGG (Arg) codon gets mutated to TGG (Trp) or other sequences. The p53 mutational analysis in all 55 samples is shown in [Figure 1]. Of 55 samples, only one HTM was observed. The p53 mutation rate in a total of 55 samples was 98% (54/55) wild type and 2% (1/55) was heterozygous. Differences in the categorical variables including age, gender, anatomical location of the tumor, stage, histopathology, grade, tobacco usage, and treatment type between patients with and without p53 mutations were evaluated. The p53 mutations and the clinicopathological factors of all the patients are shown in [Table 1].
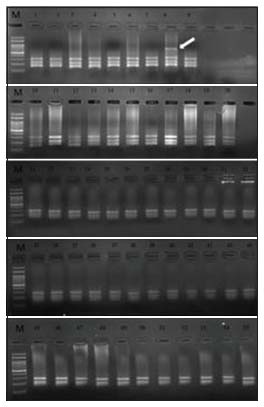
| Figure 1:Mutational analysis of p53 gene at codon 248 by polymerase chain reaction‑restriction fragment length polymorphism in all the 55 samples. M = Molecular weight marker of 100 bp. Lane 1–7 and 9–55 = Wild type and Lane 8 = heterozygous mutant (indicated by an arrow)
|
Total (n=55) |
WT (n=54) |
HTM (n=1) |
|
|---|---|---|---|
|
WT – Wild type; HTM – Heterozygous mutant; OP – Operation; RT – Radiation technology |
|||
|
Age (years) |
|||
|
<45> |
25 (45.5) |
41 (76) |
1 (100) |
|
>45 |
30 (54.5) |
13 (24) |
0 |
|
Gender |
|||
|
Male |
50 (91) |
51 (94) |
1 (100) |
|
Female |
5 (9) |
3 (6) |
0 |
|
Diagnosis |
|||
|
Oral cavity |
47 (85.5) |
42 (78) |
0 |
|
Oropharynx |
2 (3.5) |
4 (7) |
1 (100) |
|
Hypopharynx |
1 (2) |
5 (9) |
0 |
|
Larynx |
1 (2) |
3 (6) |
0 |
|
Others |
4 (7) |
0 |
0 |
|
Stage |
|||
|
Stage I |
8 (14.5) |
4 (7) |
0 |
|
Stage II |
0 |
31 (57) |
0 |
|
Stage III |
14 (25.5) |
16 (30) |
0 |
|
Stage IV a |
21 (38) |
3 (6) |
1 (100) |
|
Stage IV b |
10 (18) |
0 |
0 |
|
Stage IV c |
2 (4) |
0 |
0 |
|
Histopathology report |
|||
|
Squamous cell carcinoma |
54 (98) |
49 (91) |
1 (100) |
|
Adenocarcinoma |
1 (2) |
5 (9) |
0 |
|
Others |
0 |
0 |
0 |
|
Grade |
|||
|
Grade I |
8 (14) |
19 (35) |
1 (100) |
|
Grade II |
45 (82) |
35 (65) |
0 |
|
Grade III |
2 (4) |
0 |
0 |
|
Tobacco usage |
|||
|
Yes |
42 (76) |
48 (89) |
1 (100) |
|
No |
13 (24) |
6 (11) |
0 |
|
Treatment type |
|||
|
Radical surgery |
2 (3.3) |
53 (98) |
0 |
|
Surgery + post-OP RT |
51 (93.4) |
1 (2) |
1 (100) |
|
Radical RT |
2 (3.3) |
0 |
0 |

| Figure 1:Mutational analysis of p53 gene at codon 248 by polymerase chain reaction‑restriction fragment length polymorphism in all the 55 samples. M = Molecular weight marker of 100 bp. Lane 1–7 and 9–55 = Wild type and Lane 8 = heterozygous mutant (indicated by an arrow)
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24: 2137-50
- Rebecca LS, Kimberly DM, Ahmedin J. Cancer Statistics. Ca Cancer J Clin 2017; 70: 7-30
- Ge Z, Zhiyi L, Jeffrey NM. TP53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cell Biochem 2016; 117: 2682-92
- David PL, Sam B. p53: Oncogene or anti-oncogene. Genes Develop 1990; 4: 1-8
- Levine AJ, Perry ME, Chang A, Silver A, Dittmer D, Wu M, Welsh D. The 1993 walter hubert lecture: The role of the p53 tumour-suppressor gene in tumorigenesis. Br J Cancer 1994; 69: 409-16
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science 1991; 253: 49-53
- Field JK, Pavelic ZP, Spandidos DA, Stambrook PJ, Jones AS, Gluckman JL. The role of the p53 tumor suppressor gene in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 1993; 119: 1118-22
- Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010; 2: a001008
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015; 517: 576-82
- Caamano J, Zhang SY, Rosvold EA, Bauer B, Klein-Szanto AJP. p53 Alterations in human squamous cell carcinomas and carcinoma cell lines. Am J Pathol 1993; 142: 1131-9
- Liming O, Chongtao G, Haizhen W, Suxia L, Huizhan Z. PCR-RFLP to detect codon 248 mutation in exon 7 of p53 tumor suppressor gene. Biochem Molecular Biol Educ 2009; 37: 106-9
- Abarna R, Dutta D, Sneha P, George P, Anbalagan M. Identification of novel heterozygous Apex 1 gene variant (Glu87Gln) in patients with head and neck cancer of Indian origin. J Cell Biochem 2018; 119: 8851-61
- Debnarayan D, Rajadurai A, Mehatre S, Kannan S, Sriprakash D, Rayappa C. et al. Effect of Arg399Gln single-nucleotide polymorphism in XRCC1 gene on survival rate of Indian squamous cell head-and-neck cancer patients. J Cancer Res 2018; 20: 1-8
- Ashna G, Moorthy A. High incidence of BRAF V600 mutation in Indian patients with head and neck cancer. Front Biosci 2018; 10: 520-7
- Cara LB, Honnavara NA. p53 and the pathogenesis of skin cancer. Toxicol Appl Pharmacol 2007; 224: 241-8
- Shi Q, Xiao K, Wei W, Zhang BY, Chen C, Xu Y. et al. Associations of TP53 mutations, codon 72 polymorphism and human papillomavirus in head and neck squamous cell carcinoma patients. Oncol Rep 2013; 30: 2811-9


 PDF
PDF  Views
Views  Share
Share

