Prognostic Significance of Various Clinicopathologic Parameters and BRAF V600E Mutation in Papillary Thyroid Microcarcinoma—An Observational Study
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(03): 345-352
DOI: DOI: 10.1055/s-0043-1761412
Abstract
Introduction A specific subset of papillary microcarcinoma of thyroid (PMC) can metastasize regionally and to distant organs, and thus, have a significant effect on the overall survival of the patient cohort.
Objectives We aim to analyze the prognostic significance of various clinicopathologic parameters including BRAFV600E mutation by immunohistochemistry in PMC, in order to identify the subset of cases with aggressive behavior.
Materials and Methods Data regarding the PMC cases was retrieved retrospectively from medical records. The clinicopathologic factors like age, tumor size, focality, capsular invasion, histologic subtype, lymphovascular invasion, perithyroidal fat invasion (PTFI), lymph node (LN) metastasis, and distant metastasis were studied in depth. Tissue microarray was constructed to perform immunohistochemistry with CK19 and BRAFV600E. Information regarding overall survival (OS) and development of metastasis, if any, was noted. Chi-squared test was performed to know the association between various factors. To determine odds ratio, logistic regression was done. Survival analysis was done using Kaplan–Meier and Cox-regression analysis.
Results PMC was diagnosed in 48 patients (M:F = 1:2.4), between 22 and 70 years of age (median = 46.5 years). Chi-squared test showed significant association of fibrosis with tumor size more than or equal to 0.5 cm, infiltrative borders, PTFI, and LN metastasis. Tumor size was also associated with infiltrative borders; and LN metastasis with PTFI. BRAFV600E positivity showed significant association with histologic pattern, PTFI and distant metastasis. On logistic regression, tumor size showed significantly increased odds ratio with presence of fibrosis and infiltrative borders. Presence of fibrosis also showed significant association with infiltrative borders and LN metastasis. BRAF V600E had significantly increased odds ratio with histologic pattern, both on univariate and multivariate logistic regression. Kaplan–Meier analysis revealed significantly reduced OS with presence of LN metastases (p-value = 0.050, log-rank test). Cox-regression did not yield a significant hazard ratio for the various factors studied.
Conclusion This study shows association of LN metastasis with intratumoral fibrosis, PTFI and reduced OS. Intratumoral fibrosis was also associated with tumor size more than 5mm, infiltrative borders and PTFI. Increasing tumor size and infiltrative borders also showed an association. In addition, BRAFV600E positivity was found to be associated with histologic pattern, PTFI and distant metastasis.
Keywords
thyroid - papillary microcarcinoma - prognosis - BRAFV600E - lymph node metastasisAuthors' Contributions
Sobiya Mahnaz Ayesha contributed to designing, definition of intellectual content; literature search, experimental studies, data acquisition, data analysis, statistical analysis, manuscript preparation, editing and review; Monalisa Hui helped in designing, definition of intellectual content; literature search, experimental studies, data analysis, statistical analysis, manuscript preparation, editing and review; Shantveer G Uppin helped in conceptualization, designing, definition of intellectual content, literature search, experimental studies, data analysis, statistical analysis, manuscript preparation, editing and review; Megha Shantveer Uppin and Tara Roshni Paul helped in definition of intellectual content, manuscript preparation, editing, and review; Shubhranshu Jena, Rajsekhar Shanthappa Patil, and Ranganath Ratnagiri contributed to definition of intellectual content, clinical studies, manuscript preparation, editing, and review. Shantveer G Uppin has provided guarantee.
Ethics approval obtained from the institutional ethics committee (letter no.EC/NIMS/2809/2021).
Supplementary MaterialPublication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction A specific subset of papillary microcarcinoma of thyroid (PMC) can metastasize regionally and to distant organs, and thus, have a significant effect on the overall survival of the patient cohort.
Objectives We aim to analyze the prognostic significance of various clinicopathologic parameters including BRAFV600E mutation by immunohistochemistry in PMC, in order to identify the subset of cases with aggressive behavior.
Materials and Methods Data regarding the PMC cases was retrieved retrospectively from medical records. The clinicopathologic factors like age, tumor size, focality, capsular invasion, histologic subtype, lymphovascular invasion, perithyroidal fat invasion (PTFI), lymph node (LN) metastasis, and distant metastasis were studied in depth. Tissue microarray was constructed to perform immunohistochemistry with CK19 and BRAFV600E. Information regarding overall survival (OS) and development of metastasis, if any, was noted. Chi-squared test was performed to know the association between various factors. To determine odds ratio, logistic regression was done. Survival analysis was done using Kaplan–Meier and Cox-regression analysis.
Results PMC was diagnosed in 48 patients (M:F = 1:2.4), between 22 and 70 years of age (median = 46.5 years). Chi-squared test showed significant association of fibrosis with tumor size more than or equal to 0.5 cm, infiltrative borders, PTFI, and LN metastasis. Tumor size was also associated with infiltrative borders; and LN metastasis with PTFI. BRAFV600E positivity showed significant association with histologic pattern, PTFI and distant metastasis. On logistic regression, tumor size showed significantly increased odds ratio with presence of fibrosis and infiltrative borders. Presence of fibrosis also showed significant association with infiltrative borders and LN metastasis. BRAF V600E had significantly increased odds ratio with histologic pattern, both on univariate and multivariate logistic regression. Kaplan–Meier analysis revealed significantly reduced OS with presence of LN metastases (p-value = 0.050, log-rank test). Cox-regression did not yield a significant hazard ratio for the various factors studied.
Conclusion This study shows association of LN metastasis with intratumoral fibrosis, PTFI and reduced OS. Intratumoral fibrosis was also associated with tumor size more than 5mm, infiltrative borders and PTFI. Increasing tumor size and infiltrative borders also showed an association. In addition, BRAFV600E positivity was found to be associated with histologic pattern, PTFI and distant metastasis.
Introduction
Papillary microcarcinoma (PMC) is defined as papillary thyroid carcinoma (PTC) of size ≤1 cm.[1] Most of them are diagnosed incidentally in thyroidectomy or autopsy specimens. Advances in imaging modalities (including PET/CT, done both for screening purposes and for surveillance of other known malignancy) have led to increased early detection of small thyroid nodules.[2] [3] Many of these either remain clinically silent for the entire lifetime or have excellent long-term outcome. However, a proportion of cases metastasize to cervical lymph nodes (LNs) and distant sites. Various factors like multifocality, tumor size more than 5mm, LN metastasis and extrathyroid extension (ETE) have been found to predict the risk of recurrence.[4] [5] [6] [7] Identification of a subset of patients with aggressive behavior is important to stratify patients for implementation of radical therapeutic approach similar to classical PTC.
The frequency of BRAFV600E mutation in PTC ranges from 60 to 80%-and is associated with high risk clinicopathological features. Similar incidences have also been noted in PMC accounting for 37.5%-to 74%-of cases and are associated with aggressive features despite their small size.[8] [9] [10]
There are not many studies on PMC in Indian literature till date.[11] [12] [13] [14] [15] In this article, we have analyzed the prognostic significance of various clinicopathologic parameters including BRAFV600E mutation by immunohistochemistry (IHC) in PMC.
Materials and Methods
This is a retrospective study of cases diagnosed as PMC in our institute, from January 2014 to May 2020 (n = 48). The demographic and clinical data were retrieved form histopathology requisition forms and medical records. The inclusion criterion was thyroidectomy specimens diagnosed as PMC on routine histopathology. And exclusion criteria were tumor size exceeding 1 cm and concurrent presence of other malignancy (e.g., follicular/oncocytic carcinoma).
The hematoxylin and eosin (H&E)-stained histopathology sections of these cases were reviewed by three pathologists to confirm the original diagnosis and to also document various histopathological parameters like tumor size, focality, capsular invasion, histologic subtype, lymphovascular invasion, perithyroidal fat invasion (PTFI), and stage of the tumor. The pathological changes in the adjacent thyroid (including both neoplastic and non-neoplastic entities) were also analyzed.
Immunohistochemistry
For performing IHC, a tissue array block was constructed by extracting tumor tissue from formalin fixed paraffin embedded blocks of the cases using manual Quick-Ray needle. Of the total 48 cases, paraffin blocks of only 37 patients were available for constructing the tissue microarray and performing subsequent IHC. After initial review of H&E-stained slides, the tumor areas were marked and tissues were extracted from corresponding area on the paraffin block using 5mm tip of Quick Ray needle. The extracted tissues were then transferred to a recipient block. One or two cores were extracted per case depending on the availability of tumor tissue and each array block held 14 tumor tissue cores. Four micron sections were cut from these tissue array blocks and IHC with CK19 (Clone EP72, Pleasanton, California, USA) and BRAFV600E (Clone VE1, Ventana Hoffmann La -Roche Ltd., Switzerland) antibodies was performed on fully automated immunostainer (‘Ventana GX’ with ‘Ventana Benchmark GX, Hoffmann La - Roche Ltd, Switzerland’). The IHC results for these two antibodies performed on tissue microarray slides were analyzed. In addition, results of CK19 IHC wherever performed at time of initial diagnosis were also reviewed.
Follow-up details were noted where ever available. Information regarding overall survival, persistence of disease, and development of recurrence/metastasis if any was also noted.
Statistical Analysis
The nominal data was compared using ratios. Continuous numerical data was studied using median. Percentages were used for both nominal and ordinal data. Chi-squared test was performed to find out the association between factors like tumor size, focality, histopathological variant, infiltrative margins, fibrosis, PTFI, LN metastasis, distant metastasis, and BRAFV600E positivity. To determine odds ratio, univariate and multivariate logistic regression was done. Kaplan–Meier survival analysis was done to compare time to death between various prognostically significant groups. Cox-regression analysis (univariate and multivariate) was done to know the association of survival time with covariates and to calculate the hazard ratios for each variable. These were done using Statistical Package for the Social Sciences (SPSS) software version 29.
Ethics
Ethics approval was obtained from the NIMS institutional ethics committee (approval letter no.EC/NIMS/2809/2021, dated 28.08.2021). Waiver of informed consent was obtained due to the retrospective nature of the study. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional) and with the Helsinki declaration of 1964, as reviewed in 2013.
Results
Of the total 1,088 thyroidectomy specimens received during study period, 32.4%-(353) had malignant tumors. Of these, 58.6%-(207) were PTCs and 14.4%-(51) PMCs. Among the PMC cases, three cases were excluded as they had a history of concurrent malignancy like follicular carcinoma, oncocytic (Hurthle cell) carcinoma and mucoepidermoid carcinoma.
Demographic and Clinical Findings
The remaining 48 patients diagnosed with PMC included 29.2% (14) men and 70.8% (34) women (M:F = 1:2.4) aged between 22 and 70 years (median, 46.5 years). Of these, only 22.9% (11) presented with palpable solitary nodule in thyroid. In 43.7%-(21) patients, tumors were incidentally detected either at ultrasonography of neck (14.6%,7-cases) or thyroidectomies for other causes (multinodular goiter—27%, 13 cases; Grave's with compressive symptoms—2%, 1 case). The remaining 33.3%-(16) patients presented with metastasis in cervical LN (20.8%,10 cases) and distant sites (12.5%,6 cases). In the later cases, PMCs were detected in subsequent thyroidectomies.
Surgical Management
The surgical management in these patients included hemithyroidectomy in 29.2% (14), subtotal thyroidectomy in 20.8% (10), near total thyroidectomy in 6.25%- (3), and total thyroidectomy in 43.75% (21). Concurrent neck LN dissection was done in 39.6% (19) of these patients. Three patients who had initial hemithyroidectomy subsequently underwent completion thyroidectomy.
Pathological Findings ([Fig. 1], [Table 1])
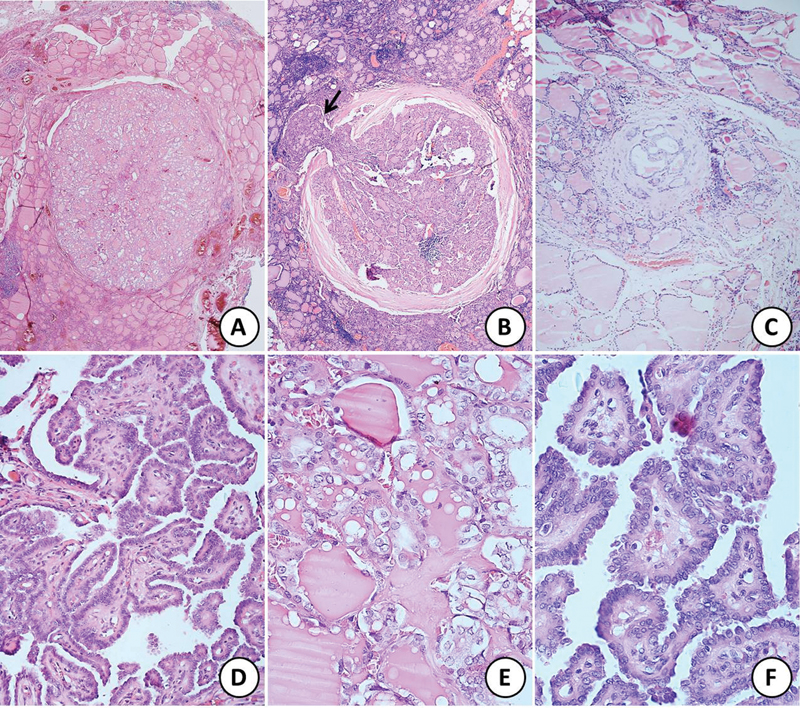
| Fig 1 :Sections of papillary microcarcinoma of thyroid showing (A) unencapsulated tumor, (B) encapsulated tumor with focal capsular breach and extracapsular extension (black arrow), and (C) very tiny focus of tumor comprising only a few follicles. All the three above tumors show follicular pattern of growth (hematoxylin and eosin [H&E]; Ax20, Bx40, Cx100). (D) Papillary microcarcinoma with classical papillary pattern of growth (H&E; x200). (E) and (F) Higher magnification to highlight the nuclear features of clearing, grooves, intranuclear inclusions, and overlapping (H&E; Dx400, Ex400).
|
Feature |
No of cases |
|
|---|---|---|
|
Focality |
Unifocal (1) Multifocal (2–6) |
30 18 |
|
Location |
Right lobe Left lobe Isthmus Bilateral lobes Isthmus+ right lobe |
23 14 5 5 1 |
|
Capsule (no. of foci = 81) |
Encapsulated: With capsular invasion Without capsular invasion Unencapsulated: Circumscribed Infiltrative |
12 3/12 9/12 69 36/69 33/69 |
|
Histologic variant (no. of foci = 81) |
Follicular variant Classic variant Tall cell variant Diffuse sclerosing variant |
50 28 2 1 |
|
Adjacent thyroid |
Calcification Psammoma bodies Fibrosis/ sclerosis Stromal hyaline Lymphocytic thyroiditis Hashimoto thyroiditis Adenomatous goiter Oncocytic (Hurthle cell) adenoma |
7 1 6/10 1 12 10 9 2 |
|
Lymph node dissection ( n = 24) |
Involved Size of largest metastatic deposit Extranodal extension |
15/24 15–60 mm 3/24 |
|
TNM stage |
T1a N1a N1b M1 |
48 1 14 6 |
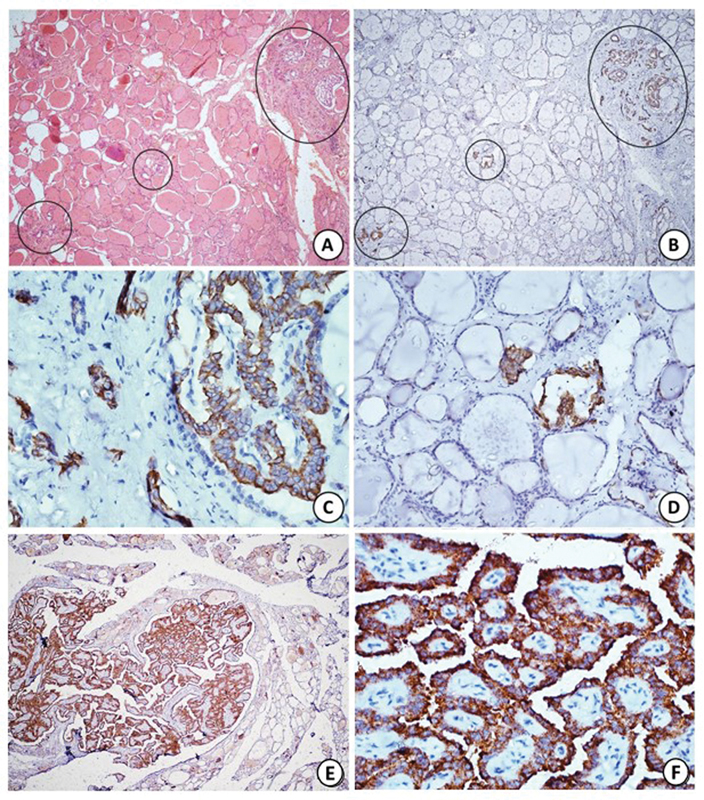
| Figure 2:(A) Section shows multicentric papillary microcarcinoma involving three distinct foci (black circles), two of which are very tiny comprising of only few follicles (hematoxylin and eosin [H&E]; x40). (B) Immunohistochemistry (IHC) with CK19 highlights all the foci of papillary microcarcinoma (black circles) including the tiny ones. (C, D) Higher magnification shows strong cytoplasmic staining in tumor cells with CK19 (HRP-Polymer; Bx40, Cx400, Dx200). (E, F) Diffuse strong cytoplasmic staining with BRAF V600E on IHC (Ex40; FX400).
Of the 77%-(37) cases included in tissue array block, IHC findings of only 73%-(35) cases could be evaluated due to tissue loss in two cases. In addition, there were four cases in which IHC results of CK19 performed at the time of original diagnosis were available for review. Thus, final results of IHC analysis are derived from 81.25%-(39) cases for CK19 and 73%-(35) cases for BRAFV600E . CK 19 was positive in all (100%, 39/39) and BRAFV600E in 54.3%-(19/35) cases tested.
Follow-Up
Follow-up details were available in 68.75%-(33) cases and the follow-up period ranged from 2 to 54 months (mean, 23.3 months). Five (10.4%) patients received radioactive iodine therapy following surgery. During follow-up, 9%-(3/33) patients developed metastases to brain (3%, 1), lung (3%, 1), and LN (3%, 1) at 5 months, 6 months, and 39 months after initial surgery, respectively. During the follow-up period, 9%-(3/33) patients died at 2, 18, and 54 months after initial surgery. One of the deceased was a patient who had brain metastasis at follow-up and died at 18 months after initial surgery.
Statistical Analysis
The chi-squared test showed significant association between presence of fibrosis and tumor size more than or equal to 0.5 cm, infiltrative borders, PTFI and LN metastasis. Apart from presence of fibrosis, tumor size more than or equal to 0.5 cm was found to be associated with infiltrative borders. LN metastasis also showed association with PTFI. BRAFV600E positivity showed significant association with histologic pattern, PTFI and distant metastasis. ([Table 2])
|
Characteristic |
Size (cm) |
Fibrosis |
LN metastasis |
BRAF |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
<0> |
≥0.5 |
p-Value |
Pre |
abs |
p-Value |
pre |
abs |
p-Value |
pos |
neg |
p-Value |
|
|
n |
19 |
29 |
16 |
32 |
15 |
33 |
19 |
16 |
||||
|
Age≥ 45 y |
9 |
20 |
0.135 |
11 |
18 |
0.404 |
10 |
19 |
0.551 |
12 |
10 |
0.968 |
|
Size ≥ 0.5 cm |
– |
– |
– |
13 |
16 |
0.037 |
11 |
18 |
0.217 |
12 |
12 |
0.452 |
|
Multifocality |
7 |
11 |
0.939 |
5 |
13 |
0.527 |
8 |
10 |
0.127 |
8 |
6 |
0.782 |
|
Infilt borders |
5 |
19 |
0.007 |
14 |
10 |
<0> |
9 |
15 |
0.350 |
11 |
7 |
0.404 |
|
Fibrosis |
3 |
13 |
0.037 |
– |
– |
– |
9 |
7 |
0.009 |
8 |
6 |
0.782 |
|
PTFI |
1 |
3 |
0.533 |
4 |
0 |
0.002 |
4 |
0 |
0.001 |
4 |
0 |
0.021 |
|
LN mets |
4 |
11 |
0.217 |
9 |
6 |
0.009 |
– |
– |
– |
8 |
4 |
0.288 |
|
Distant mets |
2 |
4 |
0.738 |
1 |
5 |
0.355 |
2 |
4 |
0.907 |
1 |
5 |
0.037 |
|
Death |
1 |
2 |
0.822 |
1 |
2 |
0.909 |
2 |
1 |
0.133 |
1 |
1 |
0.900 |
|
BRAF positive |
7 |
12 |
0.452 |
8 |
11 |
0.782 |
8 |
11 |
0.288 |
– |
– |
– |
|
Pattern: Follicular Classic Tall cell Diff scl |
12 7 0 0 |
13 13 2 1 |
0.269 |
8 6 1 1 |
17 14 1 0 |
0.465 |
10 4 1 0 |
15 16 1 1 |
0.364 |
6 11 2 0 |
11 4 0 1 |
0.032 |
|
Current study |
Park et al[19] |
Shi et al[4] |
John et al[11] |
|
|---|---|---|---|---|
|
Male: Female |
14:34 (1:2.4) |
42: 237 (1:5.6) |
21:230 (1:10.9) |
18:59 (1:3) |
|
Median age, years |
46.5 ± 15 |
46.5 ± 11.6 |
42.9 ± 10.1b |
44.54 ± 10.5b |
|
Median diameter, cm |
0.5 ± 0.6 |
0.81 ± 0.67 |
0.11 ± 0.07b |
0.41b |
|
Multifocality (%) |
18/48 (37.5) |
50/122 (41.0) |
12/251 (4.8) |
34/77 (43.4) |
|
Extrathyroidal invasion (%) |
4/48(8.3)[a] |
145/278 (52.2) |
25/251 (10) |
3/77 (3.9) |
|
LN metastasis (%) |
15/48 (31.3) |
97/278 (34.9) |
84/251 (33.5) |
11/77 (14.2) |
|
Thyroiditis (%) |
22/48 (45.8) |
10/87 (11.5) |
40/251 (15.9)c |
8/77 (10.4) |
|
Recurrent or persistent (%) |
3/33 (9)d |
6/98 (6.1) |
6/251 (2.4) |
2/77 (2.6) |
|
Mean follow-up duration, months |
23.3 ± 17.85 |
53.4 |
45.4 ± 3.5 |
20 |
References
- Rosai J, Albores Saavedra J, Asioli S. et al. Papillary thyroid carcinoma. ln: Lloyd RV, Osamura RY, Kloppel G, Rosai J, eds. WHO Classification of Tumors of Endocrine Organs. 4th edition. Lyon: IARC; 2017: 81-91
- Corn S, Mitmaker E, Tabah R, Ciarallo A, How J. Incidental thyroid uptake on PET scanning: epidemiology, clinical significance, and management challenge. J Cancer Metastasis Treat 2021; 7: 41
- Nishimori H, Tabah R, Hickeson M, How J. Incidental thyroid “PETomas”: clinical significance and novel description of the self-resolving variant of focal FDG-PET thyroid uptake. Can J Surg 2011; 54 (02) 83-88
- Shi C, Guo Y, Lv Y. et al. Clinicopathological features and prognosis of papillary thyroid microcarcinoma for surgery and relationships with the BRAFV600E mutational status and expression of angiogenic factors. PLoS One 2016; 11 (12) e0167414
- Cooper DS, Doherty GM, Haugen BR. et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19 (11) 1167-1214 [published correction appears in Thyroid. 2010 Jun;20(6):674–5]
- Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance?. Lancet Diabetes Endocrinol 2016; 4 (11) 933-942
- Kluijfhout WP, Pasternak JD, Kwon JS. et al. Microscopic positive tumor margin does not increase the risk of recurrence in patients with T1-T2 well-differentiated thyroid cancer. Ann Surg Oncol 2016; 23 (05) 1446-1451
- Bernstein J, Virk RK, Hui P. et al. Tall cell variant of papillary thyroid microcarcinoma: clinicopathologic features with BRAF(V600E) mutational analysis. Thyroid 2013; 23 (12) 1525-1531
- Lee X, Gao M, Ji Y. et al. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol 2009; 16 (02) 240-245
- Marchetti I, Iervasi G, Mazzanti CM. et al. Detection of the BRAF(V600E) mutation in fine needle aspiration cytology of thyroid papillary microcarcinoma cells selected by manual macrodissection: an easy tool to improve the preoperative diagnosis. Thyroid 2012; 22 (03) 292-298
- John AM, Jacob PM, Oommen R, Nair S, Nair A, Rajaratnam S. Our experience with papillary thyroid microcancer. Indian J Endocrinol Metab 2014; 18 (03) 410-413
- Fonseca D, Murthy SS, Tagore R. et al. BRAF status in the variants of papillary thyroid carcinoma. Int J Head Neck Pathol. 2018; 1: 41-47
- Tagore KR, Ramineni Asok Kumar S. Unusual presentation of papillary microcarcinoma of thyroid as thigh mass. Case Rep Pathol 2011; 2011: 651701
- Nimmagadda A, Krishna Mohan VS, Manthri R, Kalawat TC. Unusual metastases in papillary microcarcinoma of thyroid. Indian J Nucl Med 2019; 34 (01) 32-34
- Karkuzhali P, Yogambal M, Kumar M. An Indian tertiary care hospital scenario of papillary carcinoma of thyroid. J Clin Diagn Res 2017; 11 (06) EC26-EC29
- Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-prognostic significance of lymph node metastasis and multifocality. Cancer 2003; 98 (01) 31-40
- Lin KD, Lin JD, Huang HS, Jeng LB, Ho YS. Skull metastasis with brain invasion from thyroid papillary microcarcinoma. J Formos Med Assoc 1997; 96 (04) 280-282
- Liou MJ, Lin JD, Chung MH, Liau CT, Hsueh C. Renal metastasis from papillary thyroid microcarcinoma. Acta Otolaryngol 2005; 125 (04) 438-442
- Park YJ, Kim YA, Lee YJ. et al. Papillary microcarcinoma in comparison with larger papillary thyroid carcinoma in BRAF(V600E) mutation, clinicopathological features, and immunohistochemical findings. Head Neck 2010; 32 (01) 38-45
- Tran B, Roshan D, Abraham E. et al. An analysis of the American Joint Committee on Cancer 8th Edition T Staging System for Papillary Thyroid Carcinoma. J Clin Endocrinol Metab 2018; 103 (06) 2199-2206
- Niemeier LA, Kuffner Akatsu H, Song C. et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer 2012; 118 (08) 2069-2077
- Choi SY, Park H, Kang MK. et al. The relationship between the BRAF(V600E) mutation in papillary thyroid microcarcinoma and clinicopathologic factors. World J Surg Oncol 2013; 11: 291
- Fakhruddin N, Jabbour M, Novy M. et al. BRAF and NRAS mutations in papillary thyroid carcinoma and concordance in BRAF mutations between primary and corresponding lymph node metastases. Sci Rep 2017; 7 (01) 4666
- Friguglietti CU, Dutenhefner SE, Brandão LG, Kulcsar MA. Classification of papillary thyroid microcarcinoma according to size and fine-needle aspiration cytology: behavior and therapeutic implications. Head Neck 2011; 33 (05) 696-701
- Miccoli P, Minuto MN, Ugolini C. et al. Intrathyroidal differentiated thyroid carcinoma: tumor size-based surgical concepts. World J Surg 2007; 31 (05) 888-894
- Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 2007; 110 (01) 38-46
- Virk RK, Van Dyke AL, Finkelstein A. et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Mod Pathol 2013; 26 (01) 62-70
- Zheng X, Wei S, Han Y. et al. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol 2013; 20 (07) 2266-2273
- Mercante G, Frasoldati A, Pedroni C. et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 2009; 19 (07) 707-716
Address for correspondence
Shantveer G Uppin, MDDepartment of Pathology5th floor, Millennium Block, NIMS, Punjagutta, Hyderabad, Telangana, 500082IndiaEmail: drsguppin@yahoo.co.inPublication History
Article published online:
12 May 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 :Sections of papillary microcarcinoma of thyroid showing (A) unencapsulated tumor, (B) encapsulated tumor with focal capsular breach and extracapsular extension (black arrow), and (C) very tiny focus of tumor comprising only a few follicles. All the three above tumors show follicular pattern of growth (hematoxylin and eosin [H&E]; Ax20, Bx40, Cx100). (D) Papillary microcarcinoma with classical papillary pattern of growth (H&E; x200). (E) and (F) Higher magnification to highlight the nuclear features of clearing, grooves, intranuclear inclusions, and overlapping (H&E; Dx400, Ex400).

| Figure 2:(A) Section shows multicentric papillary microcarcinoma involving three distinct foci (black circles), two of which are very tiny comprising of only few follicles (hematoxylin and eosin [H&E]; x40). (B) Immunohistochemistry (IHC) with CK19 highlights all the foci of papillary microcarcinoma (black circles) including the tiny ones. (C, D) Higher magnification shows strong cytoplasmic staining in tumor cells with CK19 (HRP-Polymer; Bx40, Cx400, Dx200). (E, F) Diffuse strong cytoplasmic staining with BRAF V600E on IHC (Ex40; FX400).
References
- Rosai J, Albores Saavedra J, Asioli S. et al. Papillary thyroid carcinoma. ln: Lloyd RV, Osamura RY, Kloppel G, Rosai J, eds. WHO Classification of Tumors of Endocrine Organs. 4th edition. Lyon: IARC; 2017: 81-91
- Corn S, Mitmaker E, Tabah R, Ciarallo A, How J. Incidental thyroid uptake on PET scanning: epidemiology, clinical significance, and management challenge. J Cancer Metastasis Treat 2021; 7: 41
- Nishimori H, Tabah R, Hickeson M, How J. Incidental thyroid “PETomas”: clinical significance and novel description of the self-resolving variant of focal FDG-PET thyroid uptake. Can J Surg 2011; 54 (02) 83-88
- Shi C, Guo Y, Lv Y. et al. Clinicopathological features and prognosis of papillary thyroid microcarcinoma for surgery and relationships with the BRAFV600E mutational status and expression of angiogenic factors. PLoS One 2016; 11 (12) e0167414
- Cooper DS, Doherty GM, Haugen BR. et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19 (11) 1167-1214 [published correction appears in Thyroid. 2010 Jun;20(6):674–5]
- Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance?. Lancet Diabetes Endocrinol 2016; 4 (11) 933-942
- Kluijfhout WP, Pasternak JD, Kwon JS. et al. Microscopic positive tumor margin does not increase the risk of recurrence in patients with T1-T2 well-differentiated thyroid cancer. Ann Surg Oncol 2016; 23 (05) 1446-1451
- Bernstein J, Virk RK, Hui P. et al. Tall cell variant of papillary thyroid microcarcinoma: clinicopathologic features with BRAF(V600E) mutational analysis. Thyroid 2013; 23 (12) 1525-1531
- Lee X, Gao M, Ji Y. et al. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol 2009; 16 (02) 240-245
- Marchetti I, Iervasi G, Mazzanti CM. et al. Detection of the BRAF(V600E) mutation in fine needle aspiration cytology of thyroid papillary microcarcinoma cells selected by manual macrodissection: an easy tool to improve the preoperative diagnosis. Thyroid 2012; 22 (03) 292-298
- John AM, Jacob PM, Oommen R, Nair S, Nair A, Rajaratnam S. Our experience with papillary thyroid microcancer. Indian J Endocrinol Metab 2014; 18 (03) 410-413
- Fonseca D, Murthy SS, Tagore R. et al. BRAF status in the variants of papillary thyroid carcinoma. Int J Head Neck Pathol. 2018; 1: 41-47
- Tagore KR, Ramineni Asok Kumar S. Unusual presentation of papillary microcarcinoma of thyroid as thigh mass. Case Rep Pathol 2011; 2011: 651701
- Nimmagadda A, Krishna Mohan VS, Manthri R, Kalawat TC. Unusual metastases in papillary microcarcinoma of thyroid. Indian J Nucl Med 2019; 34 (01) 32-34
- Karkuzhali P, Yogambal M, Kumar M. An Indian tertiary care hospital scenario of papillary carcinoma of thyroid. J Clin Diagn Res 2017; 11 (06) EC26-EC29
- Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-prognostic significance of lymph node metastasis and multifocality. Cancer 2003; 98 (01) 31-40
- Lin KD, Lin JD, Huang HS, Jeng LB, Ho YS. Skull metastasis with brain invasion from thyroid papillary microcarcinoma. J Formos Med Assoc 1997; 96 (04) 280-282
- Liou MJ, Lin JD, Chung MH, Liau CT, Hsueh C. Renal metastasis from papillary thyroid microcarcinoma. Acta Otolaryngol 2005; 125 (04) 438-442
- Park YJ, Kim YA, Lee YJ. et al. Papillary microcarcinoma in comparison with larger papillary thyroid carcinoma in BRAF(V600E) mutation, clinicopathological features, and immunohistochemical findings. Head Neck 2010; 32 (01) 38-45
- Tran B, Roshan D, Abraham E. et al. An analysis of the American Joint Committee on Cancer 8th Edition T Staging System for Papillary Thyroid Carcinoma. J Clin Endocrinol Metab 2018; 103 (06) 2199-2206
- Niemeier LA, Kuffner Akatsu H, Song C. et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer 2012; 118 (08) 2069-2077
- Choi SY, Park H, Kang MK. et al. The relationship between the BRAF(V600E) mutation in papillary thyroid microcarcinoma and clinicopathologic factors. World J Surg Oncol 2013; 11: 291
- Fakhruddin N, Jabbour M, Novy M. et al. BRAF and NRAS mutations in papillary thyroid carcinoma and concordance in BRAF mutations between primary and corresponding lymph node metastases. Sci Rep 2017; 7 (01) 4666
- Friguglietti CU, Dutenhefner SE, Brandão LG, Kulcsar MA. Classification of papillary thyroid microcarcinoma according to size and fine-needle aspiration cytology: behavior and therapeutic implications. Head Neck 2011; 33 (05) 696-701
- Miccoli P, Minuto MN, Ugolini C. et al. Intrathyroidal differentiated thyroid carcinoma: tumor size-based surgical concepts. World J Surg 2007; 31 (05) 888-894
- Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 2007; 110 (01) 38-46
- Virk RK, Van Dyke AL, Finkelstein A. et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Mod Pathol 2013; 26 (01) 62-70
- Zheng X, Wei S, Han Y. et al. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol 2013; 20 (07) 2266-2273
- Mercante G, Frasoldati A, Pedroni C. et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 2009; 19 (07) 707-716


 PDF
PDF  Views
Views  Share
Share

