Profile of Acute Lymphoblastic Leukemia in Children Under 2 Years of Age
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(03): 307-311
DOI: DOI: 10.4103/ijmpo.ijmpo_10_17
Abstract
Context: Acute lymphoblastic leukemia (ALL) shows substantial differences in clinical and laboratory features and treatment responsiveness in different subgroups. Pediatric ALL <2 class="b" xss=removed>Aims: This study aims to analyze clinical, hematological, biochemical, immunophenotypical parameters, and treatment responsiveness of ALL under 2 years. Settings and Designs: It is a retrospective data analysis conducted at a Tertiary Care Cancer Centre in South India. The study population includes all pediatric ALL, registered at this institute during January 2009 to December 2013, who were under 2 years at the time of presentation. Materials and Methods: There were 122 cases of ALL under 2 years of age of whom 48 refused treatment, and four were lost to follow-up. Thus, 70 cases are eligible for analysis. Details on clinical, hematological, biochemical, radiological, immunophenotypical, and cytogenetic features were collected from case records, hospital cancer registry, and analysis done using SPSS version 20. Kaplan–Meier curves plotted for survival analysis. Results:: Male:female ratio was 1.26:1 (infants - 1:1; 1–2 years group - 1.3:1). The most common clinical features were hepatomegaly (95%) and fever (89%). Hemoglobin level >11 gm% was seen in 12%, WBC counts above 50,000 in 26% and platelets below 20,000 in 20%. Elevated lactate dehydrogenase presents in 64% and uric acid in 12%. Immunophenotype done in 44 children; precursor-B ALL-41, precursor-T ALL-3. Central nervous system -positive disease and 11q23 translocation were noted in one infant each. Conclusions: Infants with ALL are associated with poor prognosis. Children in the 1–2 years are associated with significantly better outcome. WBC counts >50,000 are associated with poor prognosis among infants. Treatment refusal/abandonment rate is 43% in the study population which is comparable to that in literature.
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context: Acute lymphoblastic leukemia (ALL) shows substantial differences in clinical and laboratory features and treatment responsiveness in different subgroups. Pediatric ALL <2 class="b" xss=removed>Aims: This study aims to analyze clinical, hematological, biochemical, immunophenotypical parameters, and treatment responsiveness of ALL under 2 years. Settings and Designs: It is a retrospective data analysis conducted at a Tertiary Care Cancer Centre in South India. The study population includes all pediatric ALL, registered at this institute during January 2009 to December 2013, who were under 2 years at the time of presentation. Materials and Methods: There were 122 cases of ALL under 2 years of age of whom 48 refused treatment, and four were lost to follow-up. Thus, 70 cases are eligible for analysis. Details on clinical, hematological, biochemical, radiological, immunophenotypical, and cytogenetic features were collected from case records, hospital cancer registry, and analysis done using SPSS version 20. Kaplan–Meier curves plotted for survival analysis. Results:: Male:female ratio was 1.26:1 (infants - 1:1; 1–2 years group - 1.3:1). The most common clinical features were hepatomegaly (95%) and fever (89%). Hemoglobin level >11 gm% was seen in 12%, WBC counts above 50,000 in 26% and platelets below 20,000 in 20%. Elevated lactate dehydrogenase presents in 64% and uric acid in 12%. Immunophenotype done in 44 children; precursor-B ALL-41, precursor-T ALL-3. Central nervous system -positive disease and 11q23 translocation were noted in one infant each. Conclusions: Infants with ALL are associated with poor prognosis. Children in the 1–2 years are associated with significantly better outcome. WBC counts >50,000 are associated with poor prognosis among infants. Treatment refusal/abandonment rate is 43% in the study population which is comparable to that in literature.
Keywords
Acute lymphoblastic leukemia - infantile - prognosis - survivalIntroduction
Acute lymphoblastic leukemia (ALL) is hallmarked by heterogeneous characteristics and treatment responsiveness in different subtypes. Although the overall cure rate of ALL has improved significantly over the past few decades, cure rates for specific ALL subgroups show marked variation.[1],[2],[3],[4],[5],[6],[7] Age at diagnosis is identified as an important prognostic marker of pediatric ALL.[8] It has been recognized that children with ALL, <2 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_10_17#JR_9" xss=removed>9],[10],[11],[12] Approximately 2.5%–5% of ALL are infantile and is associated with increased incidence of high white blood cell (WBC) counts, bulky extramedullary disease, translocations of MLL gene at chromosome 11q23.[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20],[21],[22] This study aims at analyzing clinical, hematological, biochemical, immunophenotypical parameters, and treatment responsiveness in children under 2 years of age, who are diagnosed with ALL.
Materials and Methods
It is a retrospective data analysis conducted at a Tertiary Care Cancer Centre in South India. The study population includes all Pediatric ALL, registered at this institute from January 2009 to December 2013, who were under 2 years at the time of presentation. The details included in the study were collected from case records, hospital cancer registry, and during follow-up.
The total number of pediatric malignancies registered during the period were 2640, of whom 422 cases were under 2 years of age with ALL contributing 122 cases (29%). Among them, 48 children refused treatment due to various reasons, and four were lost to follow-up. Thus, 70 children are eligible for analysis. Common clinical features such as fever, pallor, lymph node enlargement, hepatomegaly, splenomegaly, and bleeding were noted in each patient. Hemoglobin (Hb) levels were classified into three groups such as <7>11 gm%; WBC counts into <10000>50,000; platelet counts into <20>1 lakh. The upper limit of lactate dehydrogenase (LDH) was taken as 240 U/l and that of uric acid 6 mg%. CSF study was done to look for central nervous system (CNS) involvement.
The diagnosis of ALL was based on morphological, cytochemical, and immunohistochemical features. Lymphoblasts were classified into L1, L2, or L3 based on their morphology in Wright-Giemsa stained bone marrow smears. Cytochemistry looked into blasts positive for terminal deoxynucleotidyl transferase, periodic acid–Schiff, and negative for myeloperoxidase. Immunophenotype was identified as B-cell ALL when there are at least 20% (log2) cells positive for CD19 and CD24 and negative for CD2, CD5, and CD7. Similarly, T-cell phenotype was confirmed when at least 20% (log2) cells are positive for CD2, CD5, and CD7.
The treatment protocol followed was MCP 841 consisting of induction, intense consolidation (I2A), re-induction, conventional consolidation, and maintenance. The dose of each drug used was vincristine-1.4 mg/m 2, daunomycin (30 mg/m 2), L-Asparaginase (6000U/m 2), Prednisolone (40 mg/m 2), high-dose Ara-C (2 g/m 2), cyclophosphamide (750 mg/m 2), 6-MP (75 mg/m 2), and oral Methotrexate (15 mg/m 2), respectively. Infants below 1 year were given 50% of the dose. All infants received triple IT irrespective of CNS disease status. The dose of intrathecal methotrexate was 6 mg for infants below 1 year and 8 mg for 1–2 years and that of Ara-C was 20 mg for infants and 30 mg for 1–2 years. BM examinations were done at the end of every cycle with additional CSF study after consolidation and each maintenance cycle. Follow-up was done every month in the 1st year after completion of therapy, every 2 months in the 2nd year, three monthly in the 3rd year and yearly thereafter.
The clinical, hematological, and molecular variables collected were analyzed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp, Armonk, NY). The primary outcome measured was event-free survival (EFS), starting from the date of registration. The various events considered for calculating EFS were (i) induction failure (failure to achieve remission or induction death), (ii) leukemic relapse at any site, (iii) death, and (iv) second malignancy, whichever occurred first. Kaplan–Meier curves were plotted for both age groups.
Results
Among the 70 children, infants were 12 (17%) and the remaining 58 (83%) were in the 1–2 years group. There were 39 males (56%) and 31 (44%) females, the ratio being 1.26:1. In the infants' group, there were equal number of males and females whereas males were more (1.3:1) in the 1–2 years' group. The most common clinical features were hepatomegaly, noticed in 66 children (95%) followed by fever in 62 children (89%). Other common features were pallor and splenomegaly in 57 (81.5%) each, lymphadenopathy in 52 (74%), bleeding in 9 (11.5%), and parotidomegaly in 3 (4%) [Table 1].
|
Variable |
Total (%) |
<1> |
1-2 years (%) |
|---|---|---|---|
|
Hb – Hemoglobin; CNS – Central nervous system; TC – Total WBC Count; IPT – Immunophenotype |
|||
|
Fever |
89 |
92 |
88 |
|
Pallor |
81.5 |
83 |
81 |
|
Lymphadenopathy |
74 |
33 |
82 |
|
Bleeding |
11.5 |
8 |
14 |
|
Hepatomegaly |
95 |
92 |
96 |
|
Splenomegaly |
81.5 |
58 |
86 |
|
Renomegaly |
18.5 |
33 |
15 |
|
Hb |
|||
|
<7> |
33 |
33 |
33 |
|
7-11 |
55 |
50 |
57 |
|
>11 |
12 |
17 |
10 |
|
TC |
|||
|
<10> |
38 |
17 |
43 |
|
10,000-49,000 |
36 |
42 |
35 |
|
>50,000 |
26 |
41 |
22 |
|
Platelets |
|||
|
<20> |
20 |
17 |
21 |
|
20,000-99,999 |
74.5 |
83 |
72 |
|
>100,000 |
5.5 |
0 |
7 |
|
Morphology |
|||
|
L1 |
30 |
42 |
27 |
|
L1/L2 |
64 |
58 |
66 |
|
L2 |
6 |
0 |
7 |
|
IPT |
|||
|
B-cell |
93 |
83 |
95 |
|
T-cell |
7 |
17 |
5 |
|
CNS positive: 1 case (1.4%) |
8.3 |
0 |
|
|
11q23 mutation: 1 case (1.4%) |
8.3 |
0 |
|
|
Relapse |
<1 class="i">n=12) |
1-2 years (n=58) |
|---|---|---|
|
BM – Bone marrow; CNS – Central nervous system; IC – Intra Cranial |
||
|
BM |
1 (early-1) |
11 (early-9; late-2) |
|
Testes |
0 |
1 (late) |
|
CNS |
0 |
0 |
|
No remission |
0 |
1 |
|
Induction death |
6 |
3 |
|
Death in remission 1 (sepsis) |
5 (sepsis-4; IC bleed-1) |
|
|
Survivors |
4 |
37 |
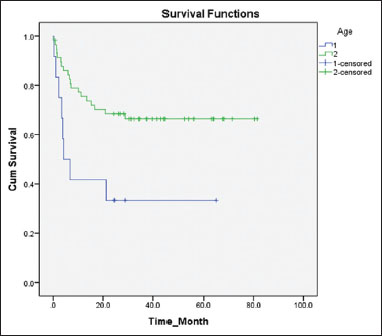
| Figure.1Comparison between the 5 years’ event‑free survival of children in two different age groups 1: Age <1>
[Table 2] shows the frequency of various events considered for the calculation of survival in each group separately. In the first group, six infants (50%) succumbed during induction, whereas there were only three induction deaths (5%) in the second group, the cumulative incidence being 12.8%. There was only one relapse (BM) in the first group (8%) when compared to the 12 relapses (19%) in the second group, of whom 11 were in the BM and one in the testis. Among the total of 13 relapses, 10 were early BM relapses, and three were late (one testicular and two BM). The number of children who died in remission while on therapy were 1 (8%) and 5 (9%) in the first and second groups, respectively. Five of them died due to septicemia and one due to intracranial hemorrhage. Only one child failed to achieve remission after induction [Figure 1].
Discussion
The incidence of organ involvement is higher in our series when compared to that in older children, with hepatomegaly being the most common (95%).[8] This observation tallies with the fact that infants and younger children who have got increased incidence of organ involvement. There is a higher proportion of children with WBC counts >50,000 as is expected in this age group. Initial WBC counts being an independent prognostic marker, this may be one of the reasons that contribute to poor prognosis of infants and young children with ALL. In our series, 18 children had a WBC count >50,000. Among them, all of the 5 infants and 5 out of the 13 in the second group succumbed, showing its clear association with dismal outcome. Although we could not establish a statistically significant difference between the survival of those with >50,000 WBC counts and those with less counts (P = 0.076), the Kaplan–Meier survival curves show that the difference in survival between the two groups is substantial [Figure 2].
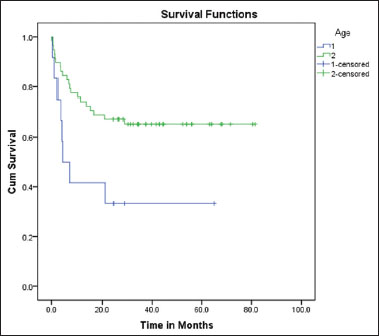
| Figure.2:Comparison between the 5 years’ event‑free survival of children with initial white blood cell counts below and above 50,000. 1: white blood cell >50,000; 2: white blood cell >50,000
Several older studies have mentioned age below 2 years as an independent prognostic marker.[9],[10],[11] Later, it was shown that the dismal prognosis is mainly associated with age <1 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_10_17#JR_9" xss=removed>9] The results obtained in our study are in line with this observation. The survival we observed in the infants' group is 33% which is consistent with that of many other studies. Although precursor T-cell ALL has a worse outcome as compared to precursor B-cell disease, our study failed to establish a significant difference between these groups.[8] This may be partly attributed to the smaller number of cases of precursor T-cell ALL.
Contrary to the belief that, the frequency of CNS-positive disease is high in the infantile ALL, we got only one case positive for CNS disease. These differences may be explained by the geographical and biological differences among the study population, as the published data are mainly from the West.
The poor prognosis associated with infantile ALL may be attributed to unique features such as high leukocyte count, increased incidence of CNS disease, massive organomegaly, CD 10 negativity, MLL gene rearrangement and vulnerability to complications and toxicities. There are very limited data to guide how the distinct physiology of infants (in terms of body composition, binding of drugs to plasma proteins, cytochrome p450 activity, renal function, and immunocompetence) should be considered in designing chemotherapy treatment protocols.[15] Initial protocols for the treatment of infant ALL have encountered problems with the excessive level of toxicities. Later, they were modified in such a way to reduce the toxicity profile and thereby the mortality.[23],[24],[25],[26]
Considering the dismal prognosis on current intensive therapies, novel therapeutic approaches, including stem cell transplantation, immunotherapy with anti-CD19 agents and FLT3 tyrosine kinase inhibitor should be incorporated into the treatment of ALL in infants along with high-quality supportive care.[15],[27],[28],[29]
Conclusions
Infants with ALL are associated with poor prognosis. Children in the 1–2 years are associated with significantly better outcome. WBC counts >50,000 are associated with poor prognosis among infants. Treatment refusal/abandonment rate is 43% in the study population which is comparable to that in literature.
Conflict of Interest
There are no conflicts of interest.
References
- Frankel LS, Ochs J, Shuster JJ, Dubowy R, Bowman WP, Hockenberry-Eaton M. et al. Therapeutic trial for infant acute lymphoblastic leukemia: The pediatric oncology group experience (POG 8493). J Pediatr Hematol Oncol 1997; 19: 35-42
- Reaman G, Zeltzer P, Bleyer WA, Amendola B, Level C, Sather H. et al. Acute lymphoblastic leukemia in infants less than one year of age: A cumulative experience of the children's cancer study group. J Clin Oncol 1985; 3: 1513-21
- Reaman GH, Steinherz PG, Gaynon PS, Bleyer WA, Finklestein JZ, Evans R. et al. Improved survival of infants less than 1 year of age with acute lymphoblastic leukemia treated with intensive multiagent chemotherapy. Cancer Treat Rep 1987; 71: 1033-8
- Ferster A, Bertrand Y, Benoit Y, Boilletot A, Behar C, Margueritte G. et al. Improved survival for acute lymphoblastic leukaemia in infancy: The experience of EORTC-childhood leukaemia cooperative group. Br J Haematol 1994; 86: 284-90
- Ishii E, Okamura J, Tsuchida M, Kobayashi M, Akiyama Y, Nakahata T. et al. Infant leukemia in Japan: Clinical and biological analysis of 48 cases. Med Pediatr Oncol 1991; 19: 28-32
- Bucsky P, Reiter A, Ritter J, Dopfer R, Riehm R. Acute lymphoblastic leukemia in infancy: Results of 5 multicenter ALL-BFM therapy studies 1970-1986. Klin Padiatr 1988; 200: 177-83
- Chessells JM, Eden OB, Bailey CC, Lilleyman JS, Richards SM. Acute lymphoblastic leukaemia in infancy: Experience in MRC UKALL trials Report from the medical research council working party on childhood leukaemia. Leukemia 1994; 8: 1275-9
- Rabin KR, Gramatges MM, Margolin JF, Poplack DG. Acute lymphoblastic leukemia In: Pizzo PA, Poplack DG editors Principles and Practice of Pediatric Oncology. Philadelphia: Wolters Kluwer 2016; 7: 463-93
- Leiper AD, Chessells J. Acute lymphoblastic leukaemia under 2 years. Arch Dis Child 1986; 61: 1007-12
- George SL, Fernbach DJ, Vietti TJ, Sullivan MP, Lane DM, Haggard ME. et al. Factors influencing survival in pediatric acute leukemia. The SWCCSG experience, 1958-1970. Cancer 1973; 32: 1542-53
- Cangir A, George S, Sullivan M. Unfavorable prognosis of acute leukemia in infancy. Cancer 1975; 36: 1973-8
- Hardisty RM, Till MM. Acute leukaemia 1959-64: Factors affecting prognosis. Arch Dis Child 1968; 43: 107-15
- Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ. et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children's oncology group. J Clin Oncol 2012; 30: 1663-9
- Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC. et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009; 360: 2730-41
- Brown P. Treatment of infant leukemias: Challenge and promise. Hematology/the education program of the American society of hematology. Am Soc Hematol Educ Program 2013; 2013: 536-600
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF. et al. SEER Cancer Statistics Review, 1975-2010. Bethesda: National Cancer Institute. 2013.
- ;Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Kremens B, Lehrnbecher T. et al. Favorable outcome in infants with AML after intensive first- and second-line treatment: An AML-BFM study group report. Leukemia 2012; 26: 654-61
- Hilden JM, Dinndorf PA, Meerbaum SO, Sather H, Villaluna D, Heerema NA. et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: Report on CCG 1953 from the children's oncology group. Blood 2006; 108: 441-51
- Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M. et al A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet 2007; 370: 240-50
- Basso G, Putti MC, Cantú-Rajnoldi A, Saitta M, Santostasi T, Santoro N. et al The immunophenotype in infant acute lymphoblastic leukaemia: Correlation with clinical outcome. An Italian multicentre study (AIEOP). Br J Haematol 1992; 81: 184-91
- ;Ludwig WD, Bartram CR, Harbott J, Köller U, Haas OA, Hansen-Hagge T. et al Phenotypic and genotypic heterogeneity in infant acute leukemia. I. Acute lymphoblastic leukemia. Leukemia 1989; 3: 431-9
- Pui CH, Ribeiro RC, Campana D, Raimondi SC, Hancock ML, Behm FG. et al. Prognostic factors in the acute lymphoid and myeloid leukemias of infants. Leukemia 1996; 10: 952-6
- Salzer WL, Jones TL, Devidas M, Hilden JM, Winick N, Hunger S. et al. Modifications to induction therapy decrease risk of early death in infants with acute lymphoblastic leukemia treated on children's oncology group P9407. Pediatr Blood Cancer 2012; 59: 834-9
- Salzer W, Jones T, Dreyer Z, Gore L, Winick N, Sung L. et al. Decreased induction morbidity and mortality with changes to induction therapy ininfants with acute lymphoblastic leukemia enrolled on children's oncology group (COG) trial AALL0631. Pediatr Blood Cancer 2012; 58: 419
- Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK. et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia 2005; 19: 2130-8
- Leung W, Hudson M, Zhu Y, Rivera GK, Ribeiro RC, Sandlund JT. et al. Late effects in survivors of infant leukemia. Leukemia 2000; 14: 1185-90
- Appelbaum FR. Allogeneic hematopoietic stem cell transplantation for acute leukemia. Semin Oncol 1997; 24: 114-23
- Uckun FM, Manivel C, Arthur D, Chelstrom LM, Finnegan D, Tuel-Ahlgren L. et al. In vivo efficacy of B43 (anti-CD19)-pokeweed antiviral protein immunotoxin against human pre-B cell acute lymphoblastic leukemia in mice with severe combined immunodeficiency. Blood 1992; 79: 2201-14
- Uckun FM, Evans WE, Forsyth CJ, Waddick KG, Ahlgren LT, Chelstrom LM. et al. Biotherapy of B-cell precursor leukemia by targeting genistein to CD19-associated tyrosine kinases. Science 1995; 267: 886-91
Address for correspondence
Publication History
Article published online:
17 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure.1Comparison between the 5 years’ event‑free survival of children in two different age groups 1: Age <1>

| Figure.2:Comparison between the 5 years’ event‑free survival of children with initial white blood cell counts below and above 50,000. 1: white blood cell >50,000; 2: white blood cell >50,000
References
- Frankel LS, Ochs J, Shuster JJ, Dubowy R, Bowman WP, Hockenberry-Eaton M. et al. Therapeutic trial for infant acute lymphoblastic leukemia: The pediatric oncology group experience (POG 8493). J Pediatr Hematol Oncol 1997; 19: 35-42
- Reaman G, Zeltzer P, Bleyer WA, Amendola B, Level C, Sather H. et al. Acute lymphoblastic leukemia in infants less than one year of age: A cumulative experience of the children's cancer study group. J Clin Oncol 1985; 3: 1513-21
- Reaman GH, Steinherz PG, Gaynon PS, Bleyer WA, Finklestein JZ, Evans R. et al. Improved survival of infants less than 1 year of age with acute lymphoblastic leukemia treated with intensive multiagent chemotherapy. Cancer Treat Rep 1987; 71: 1033-8
- Ferster A, Bertrand Y, Benoit Y, Boilletot A, Behar C, Margueritte G. et al. Improved survival for acute lymphoblastic leukaemia in infancy: The experience of EORTC-childhood leukaemia cooperative group. Br J Haematol 1994; 86: 284-90
- Ishii E, Okamura J, Tsuchida M, Kobayashi M, Akiyama Y, Nakahata T. et al. Infant leukemia in Japan: Clinical and biological analysis of 48 cases. Med Pediatr Oncol 1991; 19: 28-32
- Bucsky P, Reiter A, Ritter J, Dopfer R, Riehm R. Acute lymphoblastic leukemia in infancy: Results of 5 multicenter ALL-BFM therapy studies 1970-1986. Klin Padiatr 1988; 200: 177-83
- Chessells JM, Eden OB, Bailey CC, Lilleyman JS, Richards SM. Acute lymphoblastic leukaemia in infancy: Experience in MRC UKALL trials Report from the medical research council working party on childhood leukaemia. Leukemia 1994; 8: 1275-9
- Rabin KR, Gramatges MM, Margolin JF, Poplack DG. Acute lymphoblastic leukemia In: Pizzo PA, Poplack DG editors Principles and Practice of Pediatric Oncology. Philadelphia: Wolters Kluwer 2016; 7: 463-93
- Leiper AD, Chessells J. Acute lymphoblastic leukaemia under 2 years. Arch Dis Child 1986; 61: 1007-12
- George SL, Fernbach DJ, Vietti TJ, Sullivan MP, Lane DM, Haggard ME. et al. Factors influencing survival in pediatric acute leukemia. The SWCCSG experience, 1958-1970. Cancer 1973; 32: 1542-53
- Cangir A, George S, Sullivan M. Unfavorable prognosis of acute leukemia in infancy. Cancer 1975; 36: 1973-8
- Hardisty RM, Till MM. Acute leukaemia 1959-64: Factors affecting prognosis. Arch Dis Child 1968; 43: 107-15
- Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ. et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children's oncology group. J Clin Oncol 2012; 30: 1663-9
- Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC. et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009; 360: 2730-41
- Brown P. Treatment of infant leukemias: Challenge and promise. Hematology/the education program of the American society of hematology. Am Soc Hematol Educ Program 2013; 2013: 536-600
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF. et al. SEER Cancer Statistics Review, 1975-2010. Bethesda: National Cancer Institute. 2013.
- ;Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Kremens B, Lehrnbecher T. et al. Favorable outcome in infants with AML after intensive first- and second-line treatment: An AML-BFM study group report. Leukemia 2012; 26: 654-61
- Hilden JM, Dinndorf PA, Meerbaum SO, Sather H, Villaluna D, Heerema NA. et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: Report on CCG 1953 from the children's oncology group. Blood 2006; 108: 441-51
- Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M. et al A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet 2007; 370: 240-50
- Basso G, Putti MC, Cantú-Rajnoldi A, Saitta M, Santostasi T, Santoro N. et al The immunophenotype in infant acute lymphoblastic leukaemia: Correlation with clinical outcome. An Italian multicentre study (AIEOP). Br J Haematol 1992; 81: 184-91
- ;Ludwig WD, Bartram CR, Harbott J, Köller U, Haas OA, Hansen-Hagge T. et al Phenotypic and genotypic heterogeneity in infant acute leukemia. I. Acute lymphoblastic leukemia. Leukemia 1989; 3: 431-9
- Pui CH, Ribeiro RC, Campana D, Raimondi SC, Hancock ML, Behm FG. et al. Prognostic factors in the acute lymphoid and myeloid leukemias of infants. Leukemia 1996; 10: 952-6
- Salzer WL, Jones TL, Devidas M, Hilden JM, Winick N, Hunger S. et al. Modifications to induction therapy decrease risk of early death in infants with acute lymphoblastic leukemia treated on children's oncology group P9407. Pediatr Blood Cancer 2012; 59: 834-9
- Salzer W, Jones T, Dreyer Z, Gore L, Winick N, Sung L. et al. Decreased induction morbidity and mortality with changes to induction therapy ininfants with acute lymphoblastic leukemia enrolled on children's oncology group (COG) trial AALL0631. Pediatr Blood Cancer 2012; 58: 419
- Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK. et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia 2005; 19: 2130-8
- Leung W, Hudson M, Zhu Y, Rivera GK, Ribeiro RC, Sandlund JT. et al. Late effects in survivors of infant leukemia. Leukemia 2000; 14: 1185-90
- Appelbaum FR. Allogeneic hematopoietic stem cell transplantation for acute leukemia. Semin Oncol 1997; 24: 114-23
- Uckun FM, Manivel C, Arthur D, Chelstrom LM, Finnegan D, Tuel-Ahlgren L. et al. In vivo efficacy of B43 (anti-CD19)-pokeweed antiviral protein immunotoxin against human pre-B cell acute lymphoblastic leukemia in mice with severe combined immunodeficiency. Blood 1992; 79: 2201-14
- Uckun FM, Evans WE, Forsyth CJ, Waddick KG, Ahlgren LT, Chelstrom LM. et al. Biotherapy of B-cell precursor leukemia by targeting genistein to CD19-associated tyrosine kinases. Science 1995; 267: 886-91


 PDF
PDF  Views
Views  Share
Share

