Profile and Outcomes of COVID-19 Infection in Pediatric Patients with and without Cancer: A Case–Control Study
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(02): 150-159
DOI: DOI: 10.1055/s-0044-1786162
Abstract
Objectives Pediatric patients with cancer are considered a vulnerable population to the ill effects of coronavirus disease 2019 (COVID-19). We hereby studied the difference between clinical characteristics, lab parameters, and outcomes of COVID-19 among children suffering from cancer and those without cancer. We also analyzed risk factors for the occurrence of moderate-to-severe COVID-19 disease in pediatric cancer patients.
Materials and Methods This retrospective case–control study was carried out using the medical record review method over 6 months in a tertiary-care center in India. All patients below 18 years of age, with reverse-transcriptase polymerase chain reaction (RTPCR) confirmed COVID-19, were screened for enrolment. Patients were split into two groups: Group A comprised of patients with cancer, while group B consisted of patients without any underlying comorbidity. Patients with other comorbidity except cancer and inadequately recorded case sheets were excluded. Details regarding demography, clinical features, investigations, treatment, and outcomes were recorded.
Statistical Analysis Microsoft Excel and Statistical Package for the Social Sciences (SPSS) software, version 25 was used for data analysis. A p-value less than 0.05 was considered significant.
Results Two-hundred-five pediatric inpatients with RTPCR-established COVID-19 infection were screened and final analyses were performed on 97 patients, of which 31 children were classified into group A and 66 into group B. Median age of enrolled children was 5 years with 58.8% males. The prevalence of cancer as a comorbidity in pediatric inpatients with COVID-19 was 15%. Fifty-five percent of cancer patients had hematological malignancies, while 45% had solid tumors. Fever (p = 0.001) and gastrointestinal manifestations (p = 0.0001) were significantly less common among pediatric cancer patients. Children with cancer had significantly more leukopenia (p = 0.003), neutropenia (p = 0.003), and lymphopenia (p = 0.005). The case fatality rate was higher in children with cancer (3.2%) as compared to noncancer patients (1.5%, p = 1.0). Few risk factors for moderate-to-severe COVID-19 among children with cancer included age less than 2 years (p = 0.06), undernutrition (p = 0.33), advanced stage of cancer (p = 0.49), and presence of coinfection (p = 0.35)
Conclusion Cancer is a significant comorbidity among pediatric COVID-19 patients. While children with cancer have less severe COVID-19, their case fatality rate is higher than those without cancer. Younger age, undernutrition, advanced stage of cancer, and presence of coinfections may predispose to the development of moderate-to-severe COVID-19 among pediatric cancer patients.
Keywords
SARS-CoV-2 - pediatric cancer - hematological malignancy - risk factors - severe disease - solid tumorsAuthors' Contributions
P.K.S. conceptualized, drafted, and critically appraised the manuscript.
V.K. helped in data collection, reviewed literature, and drafted the manuscript. A.G. conceptualized, reviewed literature, and critically appraised the manuscript.
M.D., P.M., and Divyanshi helped in data collection and review of literature.
Funding
None.
Ethical Approval
Ethical approval was taken from the Institute Ethics Committee prior to commencement of this work.
Patient Consent
None declared.
Publication History
Article published online:
13 May 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Pediatric Oncology Patients and COVID-19: An Experience from the Tertiary COVID Care Facility in Eastern India: A Prospective Observational StudySatyabrata Roy Chowdhoury, Indian J Radiol Imaging, 2021
- The Impact of COVID-19 Pandemic on Cancer Care in a Tertiary Care FacilityPradeep Kumar Reddy K., South Asian Journal of Cancer, 2021
- Impact of COVID-19 Related Restrictions on Infections in Children with Cancer or after Hematopoietic SCTA Monocentric, Retrospective StudyRichard Hauch, Klinische Pädiatrie
- Clinical Profile of COVID-Positive Cancer Children across Three COVID Waves: A Tertiary Care Center's ExperienceLatha M. Sneha, VCOT Open, 2022
- The Impact of COVID-19 Pandemic on Pediatric SurgeryAnnika Mutanen, European Journal of Pediatric Surgery, 2021
- Risk and outcomes of breakthrough COVID‐19 infections in vaccinated immunocompromised patients: A meta‐analysis<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- What have we Learned about the Different COVID-19 Phenotypes in the Pediatric Population so Far?<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Elevated D-Dimer as a Marker For Thromboembolic Events in Pediatric Patients With Covid-19: A Systematic Review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- T cell cross-reactivity in autoimmune-like hepatitis triggered by COVID-19<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Quality of Life Assessment of Patients Infected With COVID-19 and Prior Coronary Artery Bypass Graft Surgery in Brazil: Four Years Follow-up<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Objectives Pediatric patients with cancer are considered a vulnerable population to the ill effects of coronavirus disease 2019 (COVID-19). We hereby studied the difference between clinical characteristics, lab parameters, and outcomes of COVID-19 among children suffering from cancer and those without cancer. We also analyzed risk factors for the occurrence of moderate-to-severe COVID-19 disease in pediatric cancer patients.
Materials and Methods This retrospective case–control study was carried out using the medical record review method over 6 months in a tertiary-care center in India. All patients below 18 years of age, with reverse-transcriptase polymerase chain reaction (RTPCR) confirmed COVID-19, were screened for enrolment. Patients were split into two groups: Group A comprised of patients with cancer, while group B consisted of patients without any underlying comorbidity. Patients with other comorbidity except cancer and inadequately recorded case sheets were excluded. Details regarding demography, clinical features, investigations, treatment, and outcomes were recorded.
Statistical Analysis Microsoft Excel and Statistical Package for the Social Sciences (SPSS) software, version 25 was used for data analysis. A p-value less than 0.05 was considered significant.
Results Two-hundred-five pediatric inpatients with RTPCR-established COVID-19 infection were screened and final analyses were performed on 97 patients, of which 31 children were classified into group A and 66 into group B. Median age of enrolled children was 5 years with 58.8% males. The prevalence of cancer as a comorbidity in pediatric inpatients with COVID-19 was 15%. Fifty-five percent of cancer patients had hematological malignancies, while 45% had solid tumors. Fever (p = 0.001) and gastrointestinal manifestations (p = 0.0001) were significantly less common among pediatric cancer patients. Children with cancer had significantly more leukopenia (p = 0.003), neutropenia (p = 0.003), and lymphopenia (p = 0.005). The case fatality rate was higher in children with cancer (3.2%) as compared to noncancer patients (1.5%, p = 1.0). Few risk factors for moderate-to-severe COVID-19 among children with cancer included age less than 2 years (p = 0.06), undernutrition (p = 0.33), advanced stage of cancer (p = 0.49), and presence of coinfection (p = 0.35)
Conclusion Cancer is a significant comorbidity among pediatric COVID-19 patients. While children with cancer have less severe COVID-19, their case fatality rate is higher than those without cancer. Younger age, undernutrition, advanced stage of cancer, and presence of coinfections may predispose to the development of moderate-to-severe COVID-19 among pediatric cancer patients.
Keywords
SARS-CoV-2 - pediatric cancer - hematological malignancy - risk factors - severe disease - solid tumorsIntroduction
Ever since the declaration of the coronavirus disease 2019 (COVID-19) pandemic, there have been many publications about COVID-19 infection in children. Most of the studies indicate that COVID-19 infection in the pediatric age group is generally mild and many remain asymptomatic. However, pediatric patients with cancer have been considered a vulnerable population to the harmful effects of COVID-19 due to their immunosuppressed state and also due to reprioritization of healthcare services.[1] To date, studies on COVID-19 in pediatric patients suffering from cancer show an asymptomatic, mild or moderate disease. However, attributable mortality in children with malignant disease is reported to be at least 10 times higher as compared to those children without comorbidities.[2] Indian data on COVID-19 in children with cancer shows low mortality due to COVID-19 infection per se.[3] However, there is limited data on the clinicoepidemiological and laboratory profile of COVID-19 in Indian children especially in the form of a case–control study.
The following research was conducted to understand the difference between clinical characteristics, lab parameters, and outcome of COVID-19 infection among pediatric patients with and without cancer. We also analyzed risk factors for the occurrence of severe COVID-19 disease in pediatric patients with cancer.
Materials and Methods
Study Design and Participants
This is a retrospective observational case–control study conducted using the medical record review method. The research was conducted after acquiring approval from the Institute's Ethics committee, over 6 months from April 2021 to September 2021, in the Department of Pediatrics of a tertiary care center in India.
Inclusion Criteria
All patients below 18 years of age with a definite diagnosis of COVID-19 through a positive nasopharyngeal and/or oropharyngeal reverse-transcriptase polymerase chain reaction (RTPCR) test were screened for enrolment.
Exclusion Criteria
Patients with any comorbidity except cancer and those with inadequately recorded case sheets were excluded.
The study cohort was split into two groups for comparison. Group A comprised pediatric patients with any form of cancer as comorbidity (newly diagnosed or already on treatment), while group B consisted of pediatric patients without any underlying comorbidity.
Primary Outcome
To analyze the difference between clinical characteristics, lab parameters, and outcome (discharge/death) of COVID-19 infection among pediatric inpatients with and without cancer.
Secondary Outcome
To identify the risk factors for the occurrence of severe COVID-19 disease in pediatric patients with cancer.
Data Collection
The following recorded data was reviewed for all enrolled patients
Demographic details: age, gender, place of current residence
Malignancy-related details: Baseline disease, disease stage, date of diagnosis, disease status (active or in remission), date of last chemotherapy/radiotherapy/surgery
COVID-19-related details: clinical manifestations, complications, disease severity at presentation, treatment received, duration of hospital stay, and outcome.
Details of coinfection: disease status, treatment received, and outcome.
The blood investigations including complete blood count, kidney function test, liver function test, coagulation profile, and radiological investigations including chest X-ray and/or computed tomography chest scan were recorded.
Outcome details: Discharge/transfer out to another facility as per existing hospital policy at that time or death.
Ethical Consideration
The research was reviewed and approved by the Institute's Ethical board, Maulana Azad Medical College & Associated Lok Nayak Hospital, GB Pant Hospital, Guru Nanak Eye Center, New Delhi-110002, registered (Registration number ECR/329/Inst/DL/2013/RR-2019) with Drug controller general of India, Directorate General of Health Services, New Delhi. All actions executed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments. Complete data anonymity was maintained.
Study Definitions
Confirmed COVID-19 Case[4]
A patient with RTPCR established severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, identified in the nasopharyngeal or oropharyngeal swab.
Disease Severity Classification[5]
Mild: Simple upper respiratory tract infection (fever, cough, sore throat, nasal congestion,) devoid of any respiratory distress and maintaining saturation of more than or equal to 95% without oxygen support. All RTPCR positive patients with isolated symptoms of fever, lassitude, or anorexia were classified into mild disease.[5]
Moderate: Pneumonia with features of fast breathing and maintaining saturation of 90-94% on room air.
Severe: Severe pneumonia with signs of fast breathing and breathlessness and maintaining saturation less than 90% on room air. In patients with severe pneumonia, the presence of the following signs was considered a severe disease: central cyanosis, feed refusal, apathy, and seizures. Acute respiratory distress syndrome, sepsis, or septic shock were considered severe diseases.
Additionally, patients with isolated gastrointestinal (GI) manifestations were classified into mild (no dehydration) or severe (severe dehydration).[6] Those with isolated central nervous system symptoms such as seizures, altered sensorium, or meningitis were classified into severe categories.[6]
Abnormal laboratory parameters were classified as follows[7]: anemia: hemoglobin less than 11 gm/dL, leukopenia: total leucocyte count (TLC) less than 4,000/ µL; leucocytosis: TLC more than 11,000 cells/ µL; neutrophilia: absolute neutrophil count (ANC) more than7,700/µL; neutropenia: ANC less than 1,500/ µL; lymphopenia: for age less than 12months, absolute lymphocyte count (ALC) less than 3000/ µL, for age more than or equal to 12 months, ALC less than 1000/ µL; lymphocytosis: for age less than 10 years, ALC more than 8,000/µL, for age more than or equal to 10 years, ALC more than 4,000/ µL; hypoalbuminemia less than 3.5 g/dL; hyperbilirubinemia more than 1.0 mg/dL elevated urea more than 40 mg/dL; elevated creatinine more than 0.9 mg/dL; elevated ferritin more than 60 ng/mL (till 9 years) and more than 300 ng/mL (10–12 years); elevated procalcitonin 0.5 ng/mL, increased IL-6 more than7 pg/mL; increased D-dimer more than 1 mg/mL.
Statistical Analysis
The data obtained from the medical records were coded into MS Excel spread sheet and evaluated using MS Excel and Statistical Package for the Social Sciences 25 (SPSS). The categorical variables were denoted as percentages and analyzed using chi-squared test or Fisher's exact test as deemed fit. Continuous variables were denoted as mean with standard deviation (normal distribution) or median with interquartile ranges (non-normal distribution). The significant difference amongst non-normally distributed variables was analyzed using Mann–Whitney U tests or Kruskal–Wallis test as necessary. A p-value less than 0.05 was taken as statistically significant.
Results
A total of 205 admitted pediatric patients with RTPCR confirmed COVID-19 infection were screened for enrolment in this study. Patients with comorbidities other than cancer (n = 59) and those with inadequately recorded case sheets (n = 49) were excluded. Final analyses were performed on 97 patients out of which 31 children were classified into group A (children with cancer) and 66 into group B (children without any comorbidity). The study flow is shown in [Fig. 1].
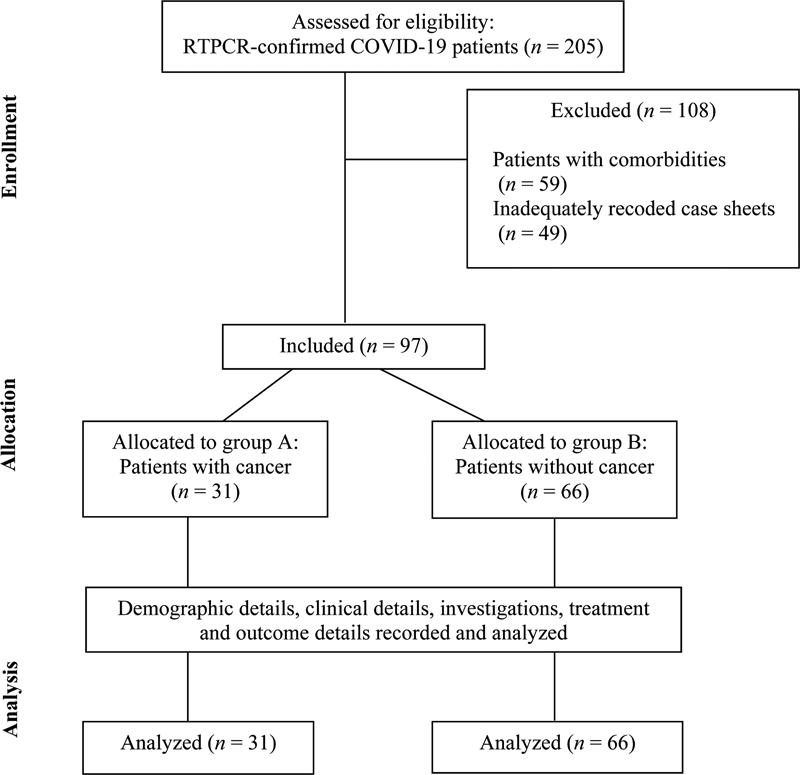
| Fig 1 : Study flow. COVID-19, coronavirus disease 2019; RTPCR, reverse-transcriptase polymerase chain reaction.
Cancer Type and Prevalence
The prevalence of cancer as a comorbidity in admitted pediatric patients with COVID-19 in this study was 15%. Overall, the median age of enrolled children was 5 years with an interquartile range of 2 to 11 years and 58.8% of them were males. The baseline characteristics, COVID-19 disease severity, and outcomes of the enrolled patients are detailed in [Table 1].
|
Parameter |
Total patients (N= 97) |
Patients with cancer (group A) (n = 31) |
Patients without comorbidity (group B) (n = 66) |
p-Value |
|---|---|---|---|---|
|
Median age in years |
5 |
6 |
5 |
|
|
(IQR) |
2–11 |
4–11 |
1.1–10.7 |
|
|
Sex (%) |
0.31 |
|||
|
Male |
57 (58.8) |
21 (67.7) |
36 (54.5) |
|
|
Female |
40 (41.2) |
10 (32.3) |
30 (45.5) |
|
|
Underweight (%) |
30 (30.9) |
15 (48.4) |
15 (22.7) |
0.01 |
|
Severity of COVID-19 (%) |
0.18 |
|||
|
Asymptomatic |
32 (33.0) |
15 (48.4) |
17 (25.7) |
|
|
Mild |
45 (46.4) |
12 (38.7) |
33 (50.0) |
|
|
Moderate |
10 (10.3) |
2 (6.4) |
8 (12.1) |
|
|
Severe |
10 (10.3) |
2 (6.4) |
8 (12.1) |
|
|
Number of patients with coinfection (%) |
15 (15.5) |
3 (9.7) |
12 (18.2) |
0.37 |
|
Duration of hospital stay in days (mean ± SD) |
10.2 ± 5.9 |
11.6 ± 6.8 |
9.6 ± 5.4 |
0.33 |
|
Outcome (%) |
1.0 |
|||
|
Death |
2 (2.0) |
1 (3.2) |
1 (1.5) |
|
|
Discharge |
95 (97.9) |
30 (96.8) |
65 (98.5) |
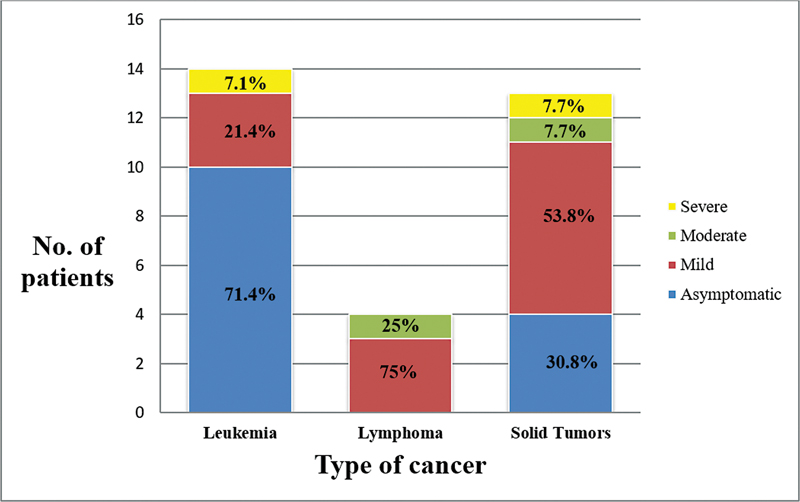
| Fig 2: Disease severity by type of cancer.
Chemotherapy Details
Out of 31 cancer patients, 14 children (45.1%) were receiving intensive chemotherapy, and 15 children (48.3%) were on nonintensive chemotherapy regimens. Two patients (6.45%) were not receiving any chemotherapy. Among the two patients, one was undergoing palliative care, while the other was yet to begin chemotherapy.
isease Severity
Amongst the enrolled patients, 33% were asymptomatic, 46.4% had mild, 10.3% had moderate, and 10.3% had severe disease overall. Group-wise details are mentioned in [Table 1].
Coinfections
Three patients (9.7%) with cancer had another coinfection (febrile neutropenia = 1, sepsis = 1, tubercular meningitis = 1), whereas 12 children without cancer (18.2%) were diagnosed to have another coinfection (disseminated tuberculosis = 2, pulmonary tuberculosis= 2, abdominal tuberculosis= 1, tubercular lymphadenitis= 1, tubercular meningitis= 1, liver abscess= 2, septic arthritis= 1, enteric fever= 1, scalp abscess = 1; p = 0.37).
Clinical Manifestations
Overall, the most common symptoms were fever (47.4%), cough (23.7%), and vomiting (16.5%). There was a significantly higher number of patients in group B who manifested with fever (p = 0.001) and vomiting (p = 0.034). Among group A, most common symptom complex at presentation was respiratory (25.8%) followed by neurological (12.9%) as compared to group B patients which showed GI (48.5%) followed by respiratory manifestations (37.9%). Group B patients had significantly higher GI manifestations (p = 0.0001). There was no statistically significant difference concerning respiratory, neurological, bleeding, and skin manifestations. A comparison of symptoms and symptom complex is detailed in [Table 2].
|
Parameter |
Total (N = 97) N (%) |
Patients with cancer (group A) (n = 31) N (%) |
Patients without comorbidity (group B) (n = 66) N (%) |
p-Value |
|---|---|---|---|---|
|
Symptoms |
||||
|
Fever |
46 (47.4) |
7 (22.6) |
39 (59.1) |
0.001 |
|
Cough |
23 (23.7) |
5 (16.1) |
18 (27.3) |
0.34 |
|
Coryza |
10 (10.3) |
3 (9.7) |
7 (10.6) |
1.0 |
|
Vomiting |
16 (16.5) |
1 (3.2) |
15 (22.7) |
0.034 |
|
Diarrhea |
10 (10.3) |
1 (3.2) |
9 (13.6) |
0.16 |
|
Pain abdomen |
6 (6.2) |
0 (0) |
6 (9.1) |
0.17 |
|
Anorexia |
2 (2.0) |
0 (0) |
2 (3.0) |
0.56 |
|
Lethargy |
4 (4.1) |
0 (0) |
4 (6.1) |
0.30 |
|
Excess irritability |
6 (6.2) |
2 (6.4) |
4 (6.1) |
1.0 |
|
Seizures |
2 (2.0) |
0 (0) |
2 (3.0) |
0.56 |
|
Headache |
7 (7.2) |
2 (6.4) |
5 (7.6) |
1.0 |
|
Fatigue |
13 (13.4) |
5 (16.1) |
8 (12.1) |
0.75 |
|
Bleeding manifestations |
2 (2.0) |
2 (6.4) |
0 (0) |
0.10 |
|
Rash |
7 (7.2) |
2 (6.4) |
5 (7.6) |
1.0 |
|
Symptom complex |
||||
|
Respiratory manifestations |
33 (34.0) |
8 (25.8) |
25 (37.9) |
0.34 |
|
GI tract manifestations |
34 (35.0) |
2 (6.4) |
32 (48.5) |
0.0001 |
|
Neurological manifestations |
19 (19.6) |
4 (12.9) |
15 (22.7) |
0.38 |
|
Bleeding manifestations |
2 (2.0) |
2 (6.4) |
0 (0) |
0.10 |
|
Skin manifestations |
7 (7.2) |
2 (6.4) |
5 (7.6) |
1.0 |
|
Parameter |
Proportion of children having abnormal values |
||
|---|---|---|---|
|
“N” observed/ “N” tested |
Patients with cancer (group A) (n = 31) |
Patients without comorbidity (group B) (n = 66) |
p-Value |
|
Anemia |
15/23 |
17/27 |
0.88 |
|
Leucopenia |
11/23 |
2/27 |
0.003 |
|
Leucocytosis |
3/23 |
10/27 |
0.10 |
|
Neutropenia |
9/23 |
1/27 |
0.003 |
|
Neutrophilia |
2/23 |
9/27 |
0.045 |
|
Lymphopenia |
11/23 |
3/27 |
0.005 |
|
Lymphocytosis |
1/23 |
2/27 |
1.0 |
|
Neutrophil: lymphocyte ratio >3.03 |
4/23 |
8/27 |
0.34 |
|
Thrombocytopenia |
10/23 |
9/27 |
0.65 |
|
Azotemia |
1/17 |
5/21 |
0.20 |
|
Hyperbilirubinemia |
3/19 |
5/20 |
0.69 |
|
Transaminitis |
11/21 |
11/20 |
0.89 |
|
Hypoalbuminemia |
5/13 |
6/11 |
0.70 |
|
Raised INR |
2/4 |
5/11 |
1.0 |
|
Raised D-dimer |
3/5 |
6/6 |
0.18 |
|
Raised interleukin-6 |
2/2 |
4/5 |
1.0 |
|
Risk factors |
Asymptomatic or mild disease N = 27 |
Moderate or severe disease N = 4 |
p-Value |
|---|---|---|---|
|
1. Age •<2> • 2–10 years • >10 year |
1 (3.7%) 16 (59.3%) 10 (37%) |
2 (50%) 1 (25%) 1 (25%) |
0.06 |
|
2. Cancer type • Solid tumors • Hematological |
11 (40.7%) 16 (59.3%) |
2 (50%) 2 (50%) |
0.56 |
|
3. Cancer stage (solid tumors and lymphomas) • Stage 1 • Stage 2 • Stage 3 • Stage 4 |
2 (15.3%) 1 (7.7%) 5 (38.5%) 5 (38.5%) |
1 (33.3%) 0 0 2 (66.7%) |
0.49 |
|
4. Cancer risk stratification (leukemias) • Standard risk • Intermediate risk • High risk |
3 (21.4%) 7 (50%) 4 (28.6%) |
0 0 1 (100%) |
0.53 |
|
5. Recent chemotherapy |
21 (77.7%) |
2 (50%) |
0.55 |
|
6. Neutrophil: lymphocyte Ratio >3.03 |
3/19 (15.8%) |
2/4 (50%) |
0.19 |
|
7. Neutropenia |
8 (42.1%) |
1 (25%) |
0.63 |
|
8. Presence of coinfection |
2 (7.4%) |
1 (25%) |
0.35 |
|
9. Underweight |
12 (44.4%) |
3 (75%) |
0.33 |
References
Address for correspondence
Publication History
Article published online:
13 May 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Pediatric Oncology Patients and COVID-19: An Experience from the Tertiary COVID Care Facility in Eastern India: A Prospective Observational StudySatyabrata Roy Chowdhoury, Indian J Radiol Imaging, 2021
- The Impact of COVID-19 Pandemic on Cancer Care in a Tertiary Care FacilityPradeep Kumar Reddy K., South Asian Journal of Cancer, 2021
- Impact of COVID-19 Related Restrictions on Infections in Children with Cancer or after Hematopoietic SCTA Monocentric, Retrospective StudyRichard Hauch, Klinische Pädiatrie
- Clinical Profile of COVID-Positive Cancer Children across Three COVID Waves: A Tertiary Care Center's ExperienceLatha M. Sneha, VCOT Open, 2022
- The Impact of COVID-19 Pandemic on Pediatric SurgeryAnnika Mutanen, European Journal of Pediatric Surgery, 2021
- Impact of the COVID-19 pandemic on time to diagnosis and treatment in children with cancer at tertiary care level<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Successful management plan of COVID-19 in a pediatric hemato-oncology department: a single-centre experience<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- 535 COVID-19 infection in childhood leukemia, management dilemma<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Impact of the COVID-19 pandemic on paediatric patients with cancer in low-income, middle-income and high-income countries: protocol for a multicentre, internati...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- COVID-19 in children with haematological malignancies<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

| Fig 1 : Study flow. COVID-19, coronavirus disease 2019; RTPCR, reverse-transcriptase polymerase chain reaction.

| Fig 2: Disease severity by type of cancer.


 PDF
PDF  Views
Views  Share
Share

