Primitive Neuroectodermal Tumor of Lung in Adult with Hemorrhagic Brain Metastasis: An Extremely Rare Case Scenario
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 84-87
DOI: DOI: 10.4103/0971-5851.203491
Abstract
Primitive neuroectodermal tumors (PNETs) are highly malignant neoplasms of embryonal origin manifesting in children and adolescents, rarely seen in adults. Carcinoma lung with hemorrhagic metastasis to the brain is very common, but primary lung PNET with hemorrhagic brain metastasis is extremely uncommon. We hereby report a 29-year-old female diagnosed as PNET lung was treated with vincristine, adriamycin, and cyclophosphamide alternating with ifosfamide plus etoposide followed by radiotherapy (RT). After 9 months, she developed hemorrhagic brain metastasis from PNET lung confirmed from tissue immunohistology postcraniotomy. Received palliative whole brain RT followed by oral pazopanib resulting in significant improvement in performance status. A thorough review of literature reveals that our case may be the second case of primary lung PNET with hemorrhagic brain metastasis and also thefirst to be exhibited oral pazopanib resulting in a significant therapeutic effect to be reported in world literature till date.
Keywords
Hemorrhagic brain metastasis - lung - pazopanib - primitive neuroectodermal tumor - whole brain radiotherapyPublication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Primitive neuroectodermal tumors (PNETs) are highly malignant neoplasms of embryonal origin manifesting in children and adolescents, rarely seen in adults. Carcinoma lung with hemorrhagic metastasis to the brain is very common, but primary lung PNET with hemorrhagic brain metastasis is extremely uncommon. We hereby report a 29-year-old female diagnosed as PNET lung was treated with vincristine, adriamycin, and cyclophosphamide alternating with ifosfamide plus etoposide followed by radiotherapy (RT). After 9 months, she developed hemorrhagic brain metastasis from PNET lung confirmed from tissue immunohistology postcraniotomy. Received palliative whole brain RT followed by oral pazopanib resulting in significant improvement in performance status. A thorough review of literature reveals that our case may be the second case of primary lung PNET with hemorrhagic brain metastasis and also the first to be exhibited oral pazopanib resulting in a significant therapeutic effect to be reported in world literature till date.
Introduction
Primitive neuroectodermal tumors (PNETs) are highly malignant embryonal neoplasms of bone and soft tissues accounting for 4%–17% of all pediatric soft tissue tumors.[1] Adult affection is extremely rare with a very dismal prognosis. Metastasis at presentation is quite common, with central PNETs metastasizing to cranium and leptomeninges while peripheral PNETs (pPNETs) disseminating to lungs, bone,[2] liver, and lymph nodes.[3] Brain is the least common site accounting for a mere 3.4%.[3] Primary pulmonary PNET is known to metastasize to pancreas, adrenal gland and ovaries[4] while it metastasizing to brain is exceedingly rare.
Case Report
A 29-year-old female presented with a cough and right-sided chest pain of 1-month duration. Chest roentgenogram revealed a nonhomogenous mass right lower zone. Computed tomography scan of chest [Figure 1] showed an 11.3 cm × 11 cm × 10 cm soft tissue mass lesion in right perihilar region with the loss of fat planes with esophagus, aorta, and diaphragm without chest wall or pleural involvement. Biopsy showed atypical round to oval cells with scanty cytoplasm intermixed with respiratory epithelium [Figure 2]. Immunohistochemistry (IHC) was positive for neuron-specific enolase (NSE) [Figure 3], synaptophysin, chromogranin, CD 99, and vimentin. Smooth muscle actin (SMA), epithelial membrane antigen (EMA), cytokeratin (CK), leukocyte common antigen (LCA), CD 20, CD 45, thyroid transcription factor (TTF-1) and desmin were negative, confirming the diagnosis of PNET lung.
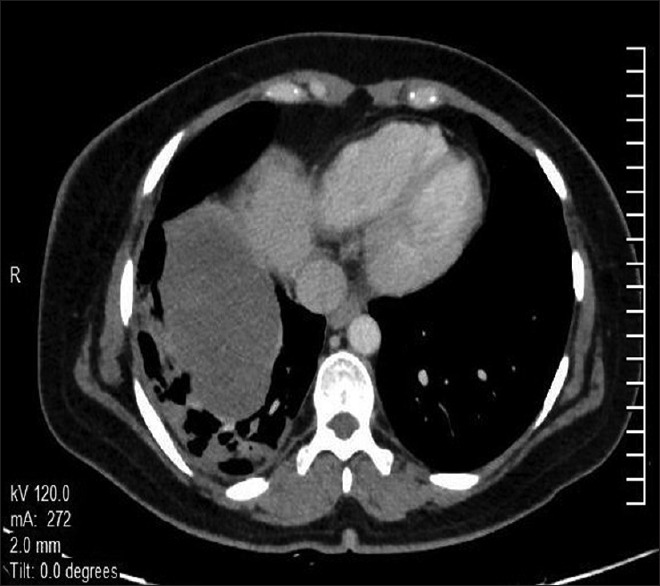
| Figure 1Computed tomography scan (axial section) of thorax showing a large heterogeneously enhancing soft tissue mass lesion in the right perihilar region involving right lower lobe extending into the mediastinum with loss of fat planes with esophagus, aorta, and right crus of the diaphragm. There is no chest wall or pleural involvement
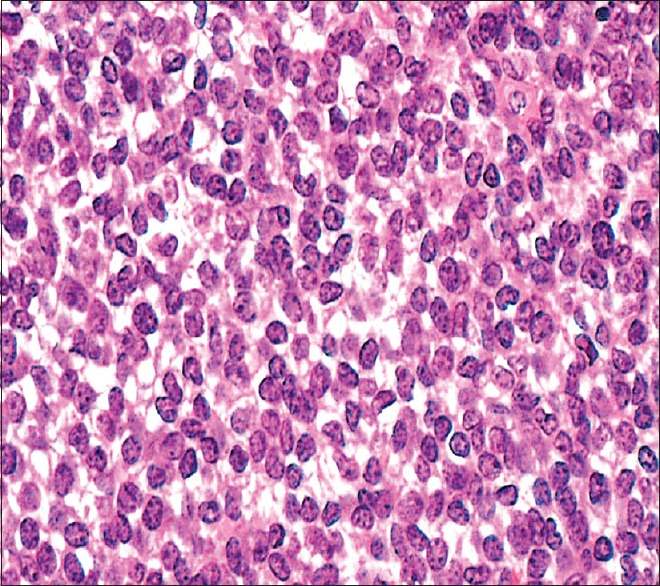
| Figure 2Biopsy from lung lesion showing small round cells with scanty cytoplasm with condensed chromatin and inconspicuous nucleoli (H and E, ×200)
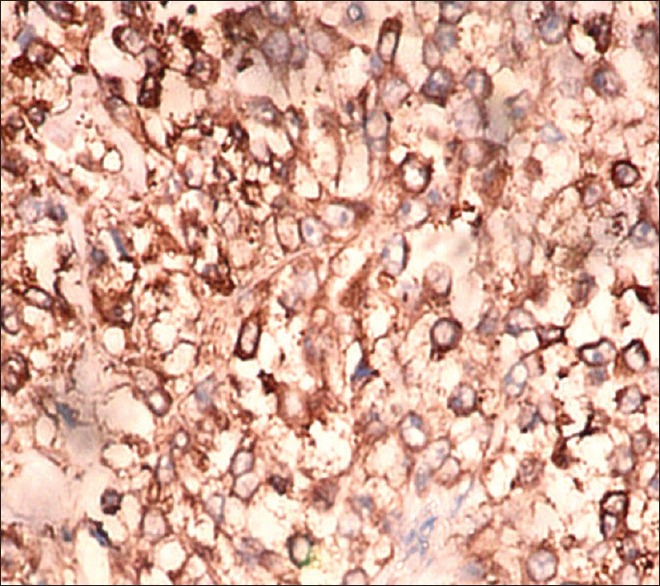
| Figure 3Immunohistochemistry picture from lung lesion showing tumor cells positive for neuron specific enolase (×200)
Magnetic resonance imaging (MRI) brain and positron emission tomography scan showed localized disease. Surgery was not considered as the lung mass invaded vital structures. Received chemotherapy vincristine, adriamycin, and cyclophosphamide alternating with ifosfamide plus etoposide (VAC/IE) resulting in partial response (PR) to therapy. Conformal radiotherapy (RT) was given to postchemotherapy residual volume to a dose of 60 (Gray) Gy in 30 fractions. Post-RT patient experienced significant symptomatic relief with improved performance status though with a static disease.
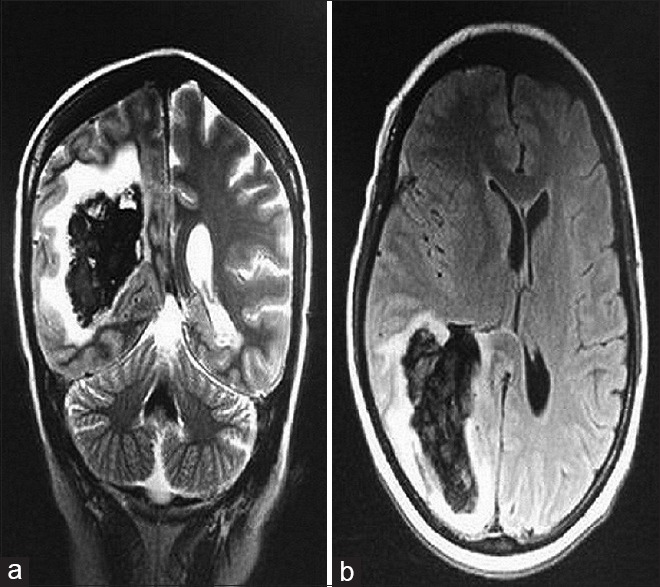
| Figure 4Magnetic resonance imaging brain (a = coronal section and b = axial section) showing a solitary lesion with areas of altered signal intensity at right periventricular and periatrial parietal lobe with significant perilesional edema suggestive of hemorrhagic metastatic deposit
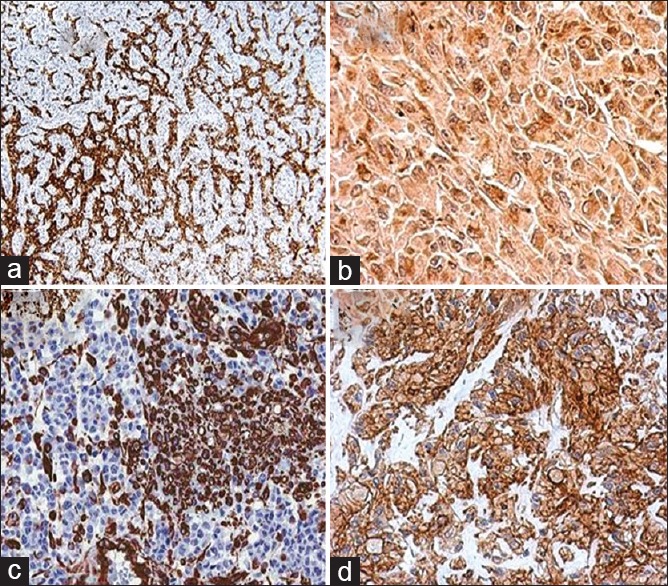
| Figure 5Immunohistochemistry picture from brain metastasis showing tumor cells positive for (a) synaptophysin (×100), (b) chromogranin (×200), (c) vimentin (×100), (d) CD 99 (×200)
Discussion
Thoracic PNETs are the most aggressive form of pPNETs arising from chest wall or underlying lung pleura invading bone, lung or mediastinum in children and young adults. They are extremely aggressive with a dismal prognosis as patients may present with an upfront metastatic disease to contralateral lung, bone,[2] bone marrow, lymph nodes, liver,[5] pancreas, adrenals, and ovaries.[4] Primary lung PNET can often be misdiagnosed with a metastatic lung lesion, mucinous adenocarcinoma or squamous cell lung carcinoma.
Since PNETs and other small round cell tumors share the same histological picture, IHC plays a vital role in their differentiation. CD 99, vimentin and S100 are nonspecific[6] while NSE, synaptophysin and chromogranin confirm the diagnosis of PNET. TTF-1 is found in pulmonary adenocarcinomas and thyroid malignancies, CK for carcinoma, desmin for rhabdomyosarcoma, SMA and EMA for soft tissue sarcoma while LCA, BCl-2, CD 20, CD 45 are for acute lymphoblastic lymphoma and leukemia. However, to confirm the diagnosis, amplification of Ewing sarcoma breakpoint region-1 by situ hybridization techniques is required.
According to Javery et al.[2] and previous other series on extraskeletal Ewing sarcoma (EES), lung is the most common site of metastasis followed by bone comprising 80% and 40%-cases, respectively. Huh et al.[3] reported lymph nodes as the most frequent metastatic site (75.9%) followed by bone, lung, peritoneum, pleura while least being brain (3.4%). The most prevalent primary sites were extremities, abdomen, pelvis, thorax, paravertebral space followed by head and neck. However, no primary lung PNET was described. Mandava et al.[7] reported a solitary case of primary pulmonary parenchymal PNET with upfront hemorrhagic brain metastasis.
Current treatment recommendations for lung PNET and pPNETs/Ewing's are same with VAC/IE.[8] RT concurrent with chemotherapy is preferred for the limited stage while sequential RT is beneficial for extensive lesions. We have treated two more cases of adult primary lung PNET although with a radiological PR but a significant symptomatic response. Both patients are on follow-up for more than a year now without any evidence of disease progression or metastasis.
For the management of brain metastasis from PNET lung, at present there is no earmarked treatment protocol. Upfront surgery was employed to provide immediate relief of mass effect. WBRT to a dose of 30 Gy was exhibited as it is the standard of care in patients with brain metastasis which has shown superiority in preventing neurologic morbidity and mortality. Pazopanib is an oral multikinase inhibitor of angiogenesis indicated mainly for progressive or chemotherapy refractory soft tissue sarcomas.[9] Although pazopanib is not the standard therapeutic option in Ewing sarcoma, it has demonstrated its efficacy in refractory and EES metastatic cases.[10]
Since Ewing's tumors and pPNETs share similar genetic characteristics with reciprocal translocations of chromosomes 11 and 22 at EWSR1, differing only in degree of neural differentiation with the former being more differentiated than latter, we, therefore, exhibited pazopanib to our patient with a palliative intent as she had progressed after standard chemotherapeutic regimen. The effectiveness of pazopanib by Children's Oncology Group (COG) NCT01956669 phase-2 study and another multi-kinase inhibitor regorafenib by NCT02048371 and NCT02389244 phase-2 studies for refractory Ewing sarcoma are underway, and the results are awaited.[10]
Newer prognostic markers should be developed to identify primary PNET lung at risk of developing brain or distant metastases. A better understanding and interpretation of the molecular and biological mechanisms of this entity may help to devise therapeutic strategies to counter the disease process. We recommend craniotomy and excision followed by WBRT for brain metastasis and oral pazopanib to check further dissemination as an incisive treatment guideline, though more prospective data favoring this recommendation is required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank the patient and his NOK for allowing us to publish the case report after his death and use the images taken during his stay in the hospital.
We also like to extend our gratitude to Departments of Pathology and Molecular Science, Surgical Oncology, Medical Oncology, Nuclear Medicine and Radiology, Army Hospital (Research and Referral), New Delhi, India.
References
- Windfuhr JP. Primitive neuroectodermal tumor of the head and neck: Incidence, diagnosis, and management. Ann Otol Rhinol Laryngol 2004;113:533-43.
- Javery O, Krajewski K, O'Regan K, Kis B, Giardino A, Jagannathan J, et al. Ato Z of extraskeletal Ewing sarcoma family of tumors in adults: Imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol 2011;197:W1015-22.
- Huh J, Kim KW, Park SJ, Kim HJ, Lee JS, Ha HK, et al. Imaging features of primary tumors and metastatic patterns of the extraskeletal Ewing sarcoma family of tumors in adults: A 17-year experience at a single institution. Korean J Radiol 2015;16:783-90.
- Shi L, Guo Z, Wu X. Primary pulmonary primitive neuroectodermal tumor metastasis to the pancreas: A rare case with seven-year follow-up. Diagn Pathol 2013;8:51.
- Somers GR, Shago M, Zielenska M, Chan HS, Ngan BY. Primary subcutaneous primitive neuroectodermal tumor with aggressive behavior and an unusual karyotype: Case report. Pediatr Dev Pathol 2004;7:538-45.
- Armbruster C, Huber M, Prosch H, Dworan N, Attems J. Ewing's sarcoma and peripheral primitive neuroectodermal tumor in adults: Different features of a rare neoplasm. Onkologie 2008;31:179-84.
- Mandava V, Sreedhar B, Pasam RK, Bala GS. Primary pulmonary primitive neuroectodermal tumor with CNS metastasis. JCR 2014;4:508-11.
- Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am 2005;19:501-25, vi-vii.
- van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879-86.
- Attia S, Okuno SH, Robinson SI, Webber NP, Indelicato DJ, Jones RL, et al. Clinical activity of pazopanib in metastatic extraosseous Ewing sarcoma. Rare Tumors 2015;7:5992.

| Figure 1Computed tomography scan (axial section) of thorax showing a large heterogeneously enhancing soft tissue mass lesion in the right perihilar region involving right lower lobe extending into the mediastinum with loss of fat planes with esophagus, aorta, and right crus of the diaphragm. There is no chest wall or pleural involvement

| Figure 2Biopsy from lung lesion showing small round cells with scanty cytoplasm with condensed chromatin and inconspicuous nucleoli (H and E, ×200)

| Figure 3Immunohistochemistry picture from lung lesion showing tumor cells positive for neuron specific enolase (×200)

| Figure 4Magnetic resonance imaging brain (a = coronal section and b = axial section) showing a solitary lesion with areas of altered signal intensity at right periventricular and periatrial parietal lobe with significant perilesional edema suggestive of hemorrhagic metastatic deposit

| Figure 5Immunohistochemistry picture from brain metastasis showing tumor cells positive for (a) synaptophysin (×100), (b) chromogranin (×200), (c) vimentin (×100), (d) CD 99 (×200)
References
- Windfuhr JP. Primitive neuroectodermal tumor of the head and neck: Incidence, diagnosis, and management. Ann Otol Rhinol Laryngol 2004;113:533-43.
- Javery O, Krajewski K, O'Regan K, Kis B, Giardino A, Jagannathan J, et al. Ato Z of extraskeletal Ewing sarcoma family of tumors in adults: Imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol 2011;197:W1015-22.
- Huh J, Kim KW, Park SJ, Kim HJ, Lee JS, Ha HK, et al. Imaging features of primary tumors and metastatic patterns of the extraskeletal Ewing sarcoma family of tumors in adults: A 17-year experience at a single institution. Korean J Radiol 2015;16:783-90.
- Shi L, Guo Z, Wu X. Primary pulmonary primitive neuroectodermal tumor metastasis to the pancreas: A rare case with seven-year follow-up. Diagn Pathol 2013;8:51.
- Somers GR, Shago M, Zielenska M, Chan HS, Ngan BY. Primary subcutaneous primitive neuroectodermal tumor with aggressive behavior and an unusual karyotype: Case report. Pediatr Dev Pathol 2004;7:538-45.
- Armbruster C, Huber M, Prosch H, Dworan N, Attems J. Ewing's sarcoma and peripheral primitive neuroectodermal tumor in adults: Different features of a rare neoplasm. Onkologie 2008;31:179-84.
- Mandava V, Sreedhar B, Pasam RK, Bala GS. Primary pulmonary primitive neuroectodermal tumor with CNS metastasis. JCR 2014;4:508-11.
- Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am 2005;19:501-25, vi-vii.
- van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879-86.
- Attia S, Okuno SH, Robinson SI, Webber NP, Indelicato DJ, Jones RL, et al. Clinical activity of pazopanib in metastatic extraosseous Ewing sarcoma. Rare Tumors 2015;7:5992.


 PDF
PDF  Views
Views  Share
Share

