Primary T-cell Lymphoblastic Lymphoma of the Ovary: A Case Report
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(01): 81-83
DOI: DOI: 10.4103/0971-5851.203501
Abstract
Primary ovarian lymphoma is extremely rare. We report a case of primary T-cell lymphoblastic lymphoma of the ovary in a 31-year-old multiparous woman, who presented with abdominal pain. Her menstrual cycles were regular. There was no generalized lymphadenopathy or fever. On per abdominal examination, there was a firm, tender, solid, mobile mass with well-defined borders, corresponding to 20 weeks gestation, whose lower pole was easily reached. Per vaginum examination revealed a large adnexal mass in the right and anterior fornix. Transabdominal ultrasonography showed bilateral solid ovarian tumor measuring 13.9 cm × 11.8 cm on the right side and 10.0 cm × 6.3 cm on the left side with significant vascularity. Tumor markers were within normal limit except for significantly elevated serum lactate dehydrogenase. Magnetic resonance imaging showed two large solid homogeneous masses, hypointense on T1W1 and hyperintense on T2W1 imaging, with a normal sized uterus and no ascites or lymphadenopathy. The patient developed one episode of left hemiparesis preoperatively, which improved spontaneously. Staging laparotomy with total abdominal hysterectomy and bilateral salpingo-oophorectomy along with infracolic omentectomy was done. Histopathology with immunohistochemistry revealed primary T-cell lymphoblastic lymphoma of the ovary, involving both ovaries left fallopian tube and left serosal surface of fundal region of uterus. She developed generalized convulsions on the 12th postoperative day, and final diagnosis was primary ovarian T-cell lymphoblastic lymphoma Ann Arbor Stage IV. She received three cycles of chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone regimen and was on palliative care. She succumbed to her illness 5½ months postoperatively.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Primary ovarian lymphoma is extremely rare. We report a case of primary T-cell lymphoblastic lymphoma of the ovary in a 31-year-old multiparous woman, who presented with abdominal pain. Her menstrual cycles were regular. There was no generalized lymphadenopathy or fever. On per abdominal examination, there was a firm, tender, solid, mobile mass with well-defined borders, corresponding to 20 weeks gestation, whose lower pole was easily reached. Per vaginum examination revealed a large adnexal mass in the right and anterior fornix. Transabdominal ultrasonography showed bilateral solid ovarian tumor measuring 13.9 cm × 11.8 cm on the right side and 10.0 cm × 6.3 cm on the left side with significant vascularity. Tumor markers were within normal limit except for significantly elevated serum lactate dehydrogenase. Magnetic resonance imaging showed two large solid homogeneous masses, hypointense on T1W1 and hyperintense on T2W1 imaging, with a normal sized uterus and no ascites or lymphadenopathy. The patient developed one episode of left hemiparesis preoperatively, which improved spontaneously. Staging laparotomy with total abdominal hysterectomy and bilateral salpingo-oophorectomy along with infracolic omentectomy was done. Histopathology with immunohistochemistry revealed primary T-cell lymphoblastic lymphoma of the ovary, involving both ovaries left fallopian tube and left serosal surface of fundal region of uterus. She developed generalized convulsions on the 12th postoperative day, and final diagnosis was primary ovarian T-cell lymphoblastic lymphoma Ann Arbor Stage IV. She received three cycles of chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone regimen and was on palliative care. She succumbed to her illness 5½ months postoperatively.
Introduction
Primary ovarian lymphoma is rare. We report a case of primary T-cell lymphoblastic lymphoma of the ovary, and discuss the challenges in the diagnosis and management of the case.
Case Report
The 31-year-old multiparous woman presented with abdominal pain of 2-month duration. Her menstrual cycles were regular, last menstrual period being 19 days back. She had two normal deliveries before, and her last childbirth was 9 years back. She was not on any contraceptives. There was no significant past or family history, including no history of familial cancers or fever. On examination, she was of average built, with no pallor or generalized lymphadenopathy. Her vitals were stable. On per abdominal examination, there was a firm, tender, solid, mobile mass of 20 weeks gestation; whose lower pole was easily reached. Per vaginum examination revealed large adnexal mass palpated in the right and anterior fornix, left fornix being free.
The patient was further worked up at our institute. Her hemoglobin was 10.9 g%, and peripheral smear, kidney function tests, and liver function tests were within normal limits. Transabdominal ultrasonography revealed bilateral solid ovarian masses [Figure 1a], measuring 13.9 cm × 11.8 cm on right side and 10.0 cm × 6.3 cm on left side, with significant vascularity on color Doppler [Figure 1b]. Her serum lactate dehydrogenase was significantly elevated, measuring 838 U/L, while CA-125 was 42.1U/ml. Other tumor markers – CA-19-9, carcinoembryonic antigen, alpha-fetoprotein, and human chorionic gonadotropin were within normal limits. Upper gastrointestinal endoscopy was normal, and stool for occult blood was negative. Computed tomography (CT) revealed two large solid masses arising from bilateral ovaries, with no ascites or lymphadenopathy [Figure 1c]. Magnetic resonance imaging (MRI) abdomen and pelvis were done for further delineation, which showed two large solid homogeneous masses, right > left, hypointense on T1W1 [Figure 1d] and hyperintense on T2W1 imaging [Figure 1e], with a normal sized uterus and no ascites. With a preoperative diagnosis of bilateral solid ovarian tumor in a young parous woman, the patient was planned for laparotomy.
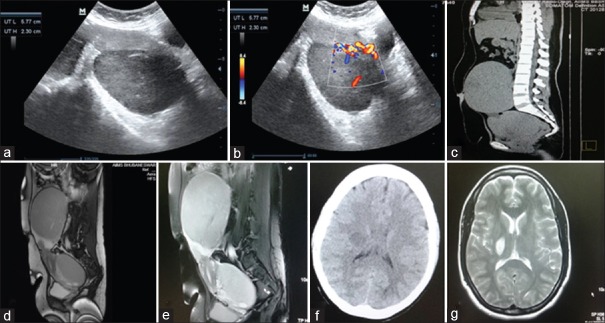
| Figure 1Figure 1 (a) Two-dimensional gray scale ultrasonography showing two solid homogeneous hypoechoic masses lying behind the uterus and separate from it, (b) Doppler ultrasonography showing significant vascularity of the mass, (c) computed tomography scan showing two large solid masses arising from bilateral ovaries, with no ascites or lymphadenopathy, (d) magnetic resonance imaging showing two large solid homogeneous masses, right > left, hypointense on T1W1 imaging, (e) magnetic resonance imaging showing two large solid homogeneous masses, right > left, hyperintense on T2W1, (f) preoperative noncontrast computed tomography brain showing showed multiple ill-defined hypodensities in the right para-sagittal, right middle cerebral artery and posterior fossa, (g) postoperative magnetic resonance imaging brain showing an increase in size of the space occupying lesion in the right subcortical (para-sagittal) area measuring 2.5 cm × 3.0 cm
However, 1 day before surgery, she developed acute onset left hemiparesis, which improved spontaneously over the next 24 h. CT brain showed multiple ill-defined hypodensities in the right parasagittal, right middle cerebral artery and posterior fossa [Figure 1f], suggestive of? infarcts/?demyelination. It was advised to go ahead with laparotomy.
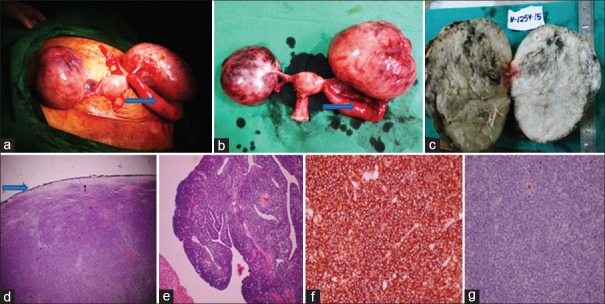
| Figure 2(a) Intraoperative finding showing enlarged bilateral ovaries, arrow pointing to enlarged left fallopian tube, (b) total abdominal hysterectomy with bilateral salpingo-oophorectomy specimen, arrow pointing to enlarged left fallopian tube, (c) cut section showing predominantly solid, homogenous, gray-white ovary with few small cysts and areas of hemorrhage, (d) on low power microscopy, ovary shows diffuse dense infiltrate of monomorphic neoplastic lymphoid cells with intact capsule (arrow), (e) low power microscopy of the left fallopian tube showing diffuse dense infiltrate of monomorphic neoplastic lymphoid cells consisting of medium-sized cells with round to oval nuclei, finely dispersed chromatin, and single to multiple small nucleoli, (f) immunohistochemistry showing tumor cells were diffusely and strongly positive for Tdt, (g) immunohistochemistry showing tumor cells were negative for B-cell marker CD-20
On immunohistochemistry, tumor cells were strongly and diffusely positive for T-cell markers-Tdt, CD-99, BCL2, and CD-10 [Figure 2f], and negative for B-cell markers – CD-20 and CD-79 [Figure 2g], with a very high proliferative index (MIB1 – 95%–100%). Bone marrow was negative for infiltration by lymphoblastic cells. Pathological diagnosis was T-cell lymphoblastic lymphoma involving both the ovaries, left fallopian tube and left serosal surface of fundal region of uterus. On day 12, she developed generalized convulsions while awaiting chemotherapy. MRI brain showed an increase in size of the space occupying lesion in the right subcortical parasagittal area measuring 2.5 cm × 3.0 cm [Figure 1g], with diffuse brain edema. Her final diagnosis was primary ovarian T-cell lymphoblastic lymphoma Ann Arbor Stage IV. She received three cycles of chemotherapy with cyclophosphamide, doxorubicin, vincristine and prednisolone regimen, and was on palliative care. She succumbed to her illness 5½ months postoperatively.
Discussion
Primary ovarian lymphoma is rare, with an incidence of 1.5% of all ovarian neoplasms, and 0.5% of all non-Hodgkin's lymphoma (NHL).[1,2] It probably arises from lymphocytes of both B- and T-cell lineage occurring within cortical granulomas, in ovarian stroma, and within ovarian follicles and corpora lutea.[3] Burkitt's lymphoma and diffuse large B-cell lymphoma are the most common histologic types seen in NHL of ovary.
Primary ovarian lymphoma is diagnosed by Fox's criteria.[4] In this case, probably due to late presentation, the extra-ovarian (brain) lesions presented within days of admission of the patient. The mean age at presentation is 47 years, with common presentation being pelvic complaints, and the size of the neoplasms ranging from 7.5 cm and 20.0 cm, mean being 13.3 cm.[5]
The preoperative imaging findings have been retrospectively described.[6,7] Ovarian lymphomas are frequently bilateral, homogeneous, without ascites, and exceeding 5 cm in diameter. Ultrasound is nonspecific with hypoechoic pattern of the ovarian mass. On CT, ovarian lymphomas appear as hypodense lesions with mild contrast enhancement. On MRI, ovarian lymphoma must be considered when large bilateral ovarian masses with a lobulated and homogeneous appearance are seen in the absence of ascites, with low signal intensity on T1W1 and mild hyperintensity on T2W1.
Image-guided core biopsy of pelvic masses has been described to be safe and accurate.[8,9,10] Primary ovarian lymphomas are staged as other extranodal NHLs using the Ann Arbor staging system. Treatment of primary ovarian lymphomas is based on histology, type, and clinical staging. Most patients have been treated by various combinations of surgery, chemotherapy, and radiotherapy.[5] However, chemotherapy is the treatment of choice, and hence, preoperative accurate diagnosis by imaging and guided biopsy helps.
Conclusion
Primary ovarian lymphomas, though rare, should be kept in mind in the differential diagnosis of solid ovarian tumors for optimal patient outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Komoto D, Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Toyama Y, et al. A case of non-Hodgkin's lymphoma of the ovary: Usefulness of 18F-FDG PET for staging and assessment of the therapeutic response. Ann Nucl Med 2006;20:157-60.
- Dimopoulos MA, Daliani D, Pugh W, Gershenson D, Cabanillas F, Sarris AH. Primary ovarian non-Hodgkin's lymphoma: Outcome after treatment with combination chemotherapy. Gynecol Oncol 1997;64:446-50.
- Chorlton I, Norris HJ, King FM. Malignant reticuloendothelial disease involving the ovary as a primary manifestation: A series of 19 lymphomas and 1 granulocystic sarcoma. Cancer 1974;34:397-407.
- Fox H, Langley FA, Govan AD, Hill AS, Bennett MH. Malignant lymphoma presenting as an ovarian tumour: A clinicopathological analysis of 34 cases. Br J Obstet Gynaecol 1988;95:386-90.
- Vang R, Medeiros LJ, Warnke RA, Higgins JP, Deavers MT. Ovarian non-Hodgkin's lymphoma: A clinicopathologic study of eight primary cases. Mod Pathol 2001;14:1093-9.
- Ferrozzi F, Catanese C, Uccelli M, Bassi P. Ovarian lymphoma. Findings with ultrasonography, computerized tomography and magnetic resonance. Radiol Med 1998;95:493-7.
- Ferrozzi F, Tognini G, Bova D, Zuccoli G. Non-Hodgkin lymphomas of the ovaries: MR findings. J Comput Assist Tomogr 2000;24:416-20.
- Thabet A, Somarouthu B, Oliva E, Gervais DA, Hahn PF, Lee SI. Image-guided ovarian mass biopsy: Efficacy and safety. J Vasc Interv Radiol 2014;25:1922-7.e1.
- Yarram SG, Nghiem HV, Higgins E, Fox G, Nan B, Francis IR. Evaluation of imaging-guided core biopsy of pelvic masses. AJR Am J Roentgenol 2007;188:1208-11.
- Yadav R, Balasundaram P, Mridha AR, Iyer VK, Mathur SR. Primary ovarian non-Hodgkin lymphoma: Diagnosis of two cases on fine needle aspiration cytology. Cytojournal 2016;13:2.

| Figure 1Figure 1 (a) Two-dimensional gray scale ultrasonography showing two solid homogeneous hypoechoic masses lying behind the uterus and separate from it, (b) Doppler ultrasonography showing significant vascularity of the mass, (c) computed tomography scan showing two large solid masses arising from bilateral ovaries, with no ascites or lymphadenopathy, (d) magnetic resonance imaging showing two large solid homogeneous masses, right > left, hypointense on T1W1 imaging, (e) magnetic resonance imaging showing two large solid homogeneous masses, right > left, hyperintense on T2W1, (f) preoperative noncontrast computed tomography brain showing showed multiple ill-defined hypodensities in the right para-sagittal, right middle cerebral artery and posterior fossa, (g) postoperative magnetic resonance imaging brain showing an increase in size of the space occupying lesion in the right subcortical (para-sagittal) area measuring 2.5 cm × 3.0 cm

| Figure 2(a) Intraoperative finding showing enlarged bilateral ovaries, arrow pointing to enlarged left fallopian tube, (b) total abdominal hysterectomy with bilateral salpingo-oophorectomy specimen, arrow pointing to enlarged left fallopian tube, (c) cut section showing predominantly solid, homogenous, gray-white ovary with few small cysts and areas of hemorrhage, (d) on low power microscopy, ovary shows diffuse dense infiltrate of monomorphic neoplastic lymphoid cells with intact capsule (arrow), (e) low power microscopy of the left fallopian tube showing diffuse dense infiltrate of monomorphic neoplastic lymphoid cells consisting of medium-sized cells with round to oval nuclei, finely dispersed chromatin, and single to multiple small nucleoli, (f) immunohistochemistry showing tumor cells were diffusely and strongly positive for Tdt, (g) immunohistochemistry showing tumor cells were negative for B-cell marker CD-20
References
- Komoto D, Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Toyama Y, et al. A case of non-Hodgkin's lymphoma of the ovary: Usefulness of 18F-FDG PET for staging and assessment of the therapeutic response. Ann Nucl Med 2006;20:157-60.
- Dimopoulos MA, Daliani D, Pugh W, Gershenson D, Cabanillas F, Sarris AH. Primary ovarian non-Hodgkin's lymphoma: Outcome after treatment with combination chemotherapy. Gynecol Oncol 1997;64:446-50.
- Chorlton I, Norris HJ, King FM. Malignant reticuloendothelial disease involving the ovary as a primary manifestation: A series of 19 lymphomas and 1 granulocystic sarcoma. Cancer 1974;34:397-407.
- Fox H, Langley FA, Govan AD, Hill AS, Bennett MH. Malignant lymphoma presenting as an ovarian tumour: A clinicopathological analysis of 34 cases. Br J Obstet Gynaecol 1988;95:386-90.
- Vang R, Medeiros LJ, Warnke RA, Higgins JP, Deavers MT. Ovarian non-Hodgkin's lymphoma: A clinicopathologic study of eight primary cases. Mod Pathol 2001;14:1093-9.
- Ferrozzi F, Catanese C, Uccelli M, Bassi P. Ovarian lymphoma. Findings with ultrasonography, computerized tomography and magnetic resonance. Radiol Med 1998;95:493-7.
- Ferrozzi F, Tognini G, Bova D, Zuccoli G. Non-Hodgkin lymphomas of the ovaries: MR findings. J Comput Assist Tomogr 2000;24:416-20.
- Thabet A, Somarouthu B, Oliva E, Gervais DA, Hahn PF, Lee SI. Image-guided ovarian mass biopsy: Efficacy and safety. J Vasc Interv Radiol 2014;25:1922-7.e1.
- Yarram SG, Nghiem HV, Higgins E, Fox G, Nan B, Francis IR. Evaluation of imaging-guided core biopsy of pelvic masses. AJR Am J Roentgenol 2007;188:1208-11.
- Yadav R, Balasundaram P, Mridha AR, Iyer VK, Mathur SR. Primary ovarian non-Hodgkin lymphoma: Diagnosis of two cases on fine needle aspiration cytology. Cytojournal 2016;13:2.


 PDF
PDF  Views
Views  Share
Share

