Primary Diffuse Large B-cell Lymphoma of the Breast: A Rare Case and Review of Literature
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 244-247
DOI: DOI: 10.4103/ijmpo.ijmpo_112_16

|
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Sir,
Primary breast lymphoma (PBL) with reported incidence of 0.05%–0.53% of all malignant diseases of the breast is a very uncommon condition and accounts for 2.2% of all extranodal malignant lymphomas.[1] The most common histology in PBL is diffuse large B-cell lymphoma (DLBCL).[2] PBL is reported to exhibit poor prognosis among extranodal B-cell lymphomas, reasons being unknown. PBL may have similar clinical and radiological presentation as primary breast carcinoma, but treatment modalities and outcomes differ. Here, we report a case of PBL who was treated with chemotherapy and obtained complete remission (CR) of her disease and also do literature review of current management patterns of PBL with DLBCL.
A 65-year-old female with no history of comorbidities was admitted to our hospital on July 5, 2014 with painless lump in her right breast. On detailed history taking, patient had no history of fever, night sweats, or weight loss (B symptoms). Physical examination revealed a hard, nontender, mobile mass of approximately 6 cm × 7 cm occupying all quadrants of the right breast. The contralateral breast was normal. On physical examination, patient had no evidence of cervical, axillary or inguinal lymphadenopathy. Liver and spleen were not palpable. The laboratory tests revealed the following: Hemoglobin of 11.9 g/dl, total leukocyte counts of 10,400/mm3, platelet counts of 48,600/mm3, serum creatinine of 0.72 mg/dl, alkaline phosphatase of 55 IU/L, serum glutamic pyruvic transaminase of 22 IU/L, serum glutamic oxaloacetic transaminase of 13 IU/L, total bilirubin levels of 0.88 mg/dl, serum albumin of 4.34 g/dl, lactate dehydrogenase (LDH) of 172 U/L, and serum uric acid of 4.52 mg/dl. Excisional biopsy performed showed atypical lymphocytic infiltration suspicious of non-Hodgkin's lymphoma (NHL) [Figure 1]. Immunohistochemistry revealed positivity to CD20 [Figure 2], CD79a and MUM1 and negativity to CD2, CD3, CD5, CD10, and AE1. MIB-1 index was 95%. On the basis of histopathologic features, tumor was classified as DLBCL, non-germinal centre B-cell-like (non-GCB DLBCL). Bone marrow aspiration and biopsy were performed and revealed a hypercellular bone marrow with no evidence of lymphomatous infiltration. The cerebrospinal fluid cytological examination was negative for any malignant cells. Computed tomography (CT) of the neck, thorax, abdomen, and pelvis revealed 66 mm × 68 mm × 84 mm large multi-lobulated soft tissue density lesion involving all quadrants of right breast reaching up to the skin [Figure 3]. No systemic lymphadenopathy was detected. The CT findings indicated stage I E of the lymphoma tumor according to the Ann Arbor staging system. The patient received six courses of cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab (R-CHOP) chemotherapy. After four courses of R-CHOP, the follow-up chest CT scan showed decreased the size of the right breast mass (6.6 cm × 6.8 cm → 3.4 cm × 1.4 cm) [Figure 4]. After six courses of R-CHOP, the follow-up chest CT scan showed no visible mass in the breast. The patient was put under close observation. At present, after follow-up period of 20 months, the patient is surviving with no evidence of disease and with no morbidities associated with chemotherapy.
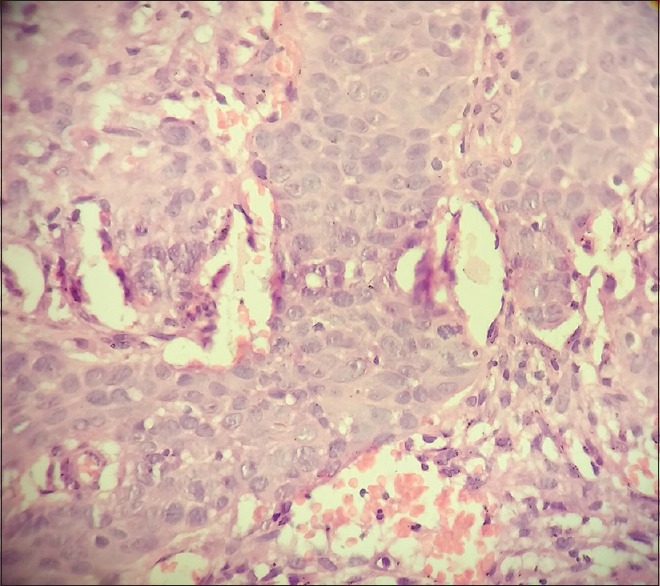
| Figure 1:H and E (×40) section showing diffuse proliferation of large cells with high mitotic rate
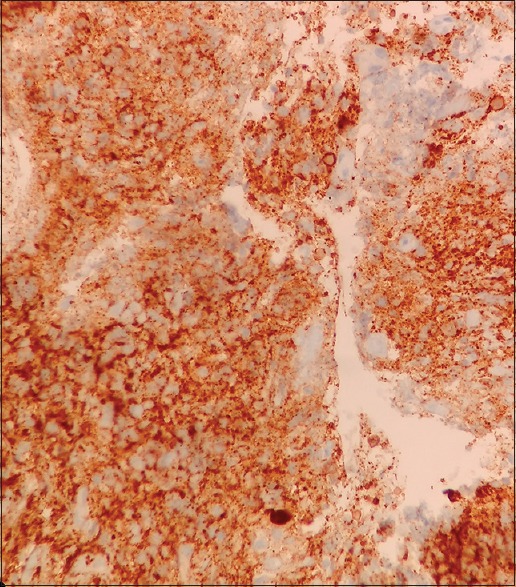
| Figure 2:Immunohistochemistry stain showing CD20 positivity of diffuse large B-cells
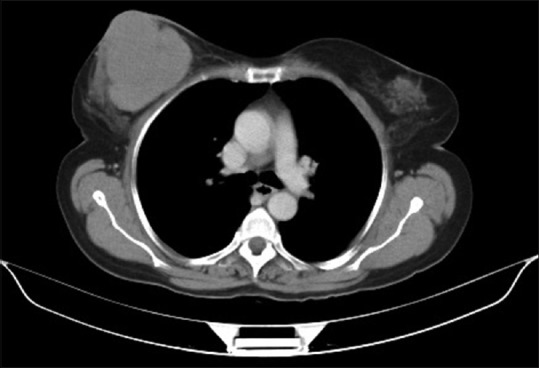
| Figure 3:Computed tomography scan showing a large multi-lobulated soft tissue density lesion involving all quadrants of right breast reaching up to the skin measuring 66 mm × 68 mm × 84 mm
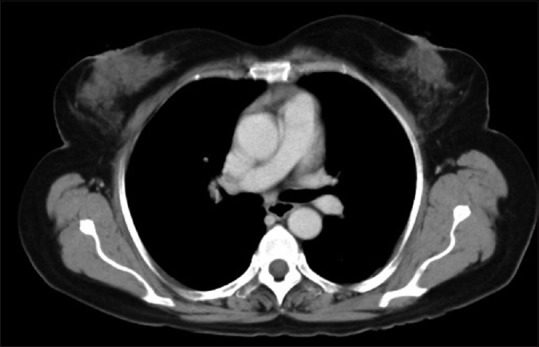
| Figure 4:Interim computed tomography scan done after four cycles of cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab showing marked reduction in size of right breast mass from 6.6 cm × 6.8 cm to 3.4 cm × 1.4 cm
DLBCL, the most common histologic subtype constituting 31% of all NHLs, includes a diffuse proliferation of large cells that have a high mitotic rate. Stage IE disease is present in approximately 20% of the patients with DLBCL. Extranodal sites occur in 40% of cases with common sites involved being gastrointestinal tract, testis, bone, thyroid, skin, central nervous system (CNS), and bone marrow.[3] PBL has reported incidence of 0.05%–0.53% of all malignant diseases of the breast and accounts for 2.2% of all extranodal malignant lymphomas.[1] PBL is defined as localized lymphoma to one or both breasts with or without regional lymph nodes such as ipsilateral axillary and/or supraclavicular lymph nodes.[4] DLBCL is the most common histopathological type of PBL, with other subtypes include marginal zone lymphoma, follicular lymphoma, mantle cell lymphoma, and Burkitt lymphoma.[5] The peak age of PBL is usually the sixth decade, as was in our case, varying among various ethnic groups with median age in the East Asian countries being approximately 10 years (45–53 years) younger than that in the Western countries (62–64 years).[6] PBL occurs more frequently in the right breast with ratio of 3:2, as was in our case.[7] Hans et al. subclassified DLBCL into GCB type and non-GCB type. DLBCL cases of CD10 (+) or CD10 (−) Bcl-6 (+) MUM1 (−) were subclassified as GCB type. CD10 (−) cases with MUM1 (+) regardless of Bcl-6 expression, were subclassified as non-GCB type.[8] Yoshida et al.[1] reported that PBL is usually of non-GCB type, portending a poor prognosis. Furthermore, Bcl-2 (+) cases showed significantly worse prognosis than Bcl-2 (−) patients in non-GCB type. MIB-1 index has a relationship with prognosis in B-cell lymphomas. DLBCL with mucosa-associated lymphoid tissue-type lymphoma in the stomach and of DLBCL in the CNS were reported to have MIB-1 index of 51% and 50%, respectively while PBL cases have MIB-1 index ranging from 60% to 95%. These data clearly indicate high proliferative activity in PBL accounting for recurrence rate even in stage IE disease. Primary DLBCL of the breast has distinct clinicopathological characteristics in that it has the propensity to recur in opposite breast, other extranodal sites and CNS. As reported by Jeanneret-Sozzi et al.[9] for PBL, the 5-year overall survival (OS) rate is 53%, lymphoma-specific survival is 59%, disease-free survival is 41%, and local control rate is 87%.
An anthracycline-based regimen is the main-stay of the treatment of PBL, with CHOP being the most frequently used regimen as in other nodal forms of DLBCL. Very few studies have been conducted to evaluate the role of rituximab in the management of PBL-DLBCL. In a study conducted by Avilés et al.,[10] CNS relapses were ameliorated with the addition of rituximab while 11% rate of CNS relapse seen in arm treated without rituximab. However, Consortium for Improving Survival of Lymphoma study failed to show a positive effect on OS and progression-free survival (PFS) with the addition of rituximab. This same study demonstrated that treatment with less than four cycles of chemotherapy had negative impact on 5-year PFS and OS.[4] The study conducted by Avilés et al.[10] was the only randomized trial using radiotherapy in primary breast-DLBCL (PB-DLBCL). In this trial patients were randomized into three arms: Radiotherapy only (45 Gy to breast and its lymphatic drainage), chemotherapy only (six cycles of CHOP every 21 days) and combined modality treatment (CMT) (six cycles of CHOP every 21 days, followed by radiotherapy of 30 Gy). This study was stopped early because an interim analysis revealed a higher CR and OS and a lower relapse rate in the CMT arm. These superior results of CMT were supported by the observations made by the International Extranodal Lymphoma Study Group (IELSG)[7] which demonstrated superior outcomes for the subgroup of patients with anthracycline-based regimens and consolidative radiotherapy. Regarding the role of surgery, the IELSG data showed that radical mastectomy was associated with an increased the risk of death. CNS relapse is noted in only 5% of PB-DLBCL patients. Some trials demonstrated that addition of rituximab to chemotherapy decreased the rate of CNS relapse.[11,12] The authors of the IELSG study concluded that routine CNS prophylaxis is not required for patients with PB-DLBCL. Our patient received six cycles of R-CHOP with excellent response, with no CNS prophylaxis or radiotherapy given. At present, patient is under surveillance for the past 20 months with no evidence of locoregional or distant relapse.
PBL lacks specific clinical and radiological diagnostic features with the majority of patients treated initially as breast cancer patients. Identification of this rare entity is important as surgery is associated with high morbidity rate. Surgery should be minimally invasive and for diagnostic purpose only: Core needle or excisional biopsy. After diagnosis and staging, use systemic therapy with at least four cycles of anthracycline and rituximab-based regimen even for stage IE disease. Radiotherapy has an important role as adjuvant consolidation therapy, particularly in node-negative (stage IE) patients. However, recommendations for optimal dose or fields of irradiation for PBL are currently unavailable. Optimal combined modality primary treatment in addition to a selection of high-risk patients may obviate the need for routine CNS prophylaxis in all cases. High-risk patients (poor performance status, elevated LDH, high international prognostic index score) may benefit from the addition of CNS prophylaxis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Yoshida S, Nakamura N, Sasaki Y, Yoshida S, Yasuda M, Sagara H, et al. Primary breast diffuse large B-cell lymphoma shows a non-germinal center B-cell phenotype. Mod Pathol 2005;18:398-405.
- Lyons JA, Myles J, Pohlman B, Macklis RM, Crowe J, Crownover RL. Treatment of prognosis of primary breast lymphoma: A review of 13 cases. Am J Clin Oncol 2000;23:334-6.
- Arnold SF, Caron AJ, Peter M, Jon CA. Non-Hodgkin's lymphoma. DeVita, Hellman, and Rosenberg's cancer: Principles & practice of oncology: 10th ed. Wolters Kluwer Health Adis (ESP); 2015. p. 1552-83.
- Yhim HY, Kang HJ, Choi YH, Kim SJ, Kim WS, Chae YS, et al. Clinical outcomes and prognostic factors in patients with breast diffuse large B cell lymphoma: Consortium for Improving Survival of Lymphoma (CISL) study. BMC Cancer 2010;10:321.
- Ganjoo K, Advani R, Mariappan MR, McMillan A, Horning S: Non-Hodgkin lymphoma of the breast. Cancer 2007;110:25-30.
- Cheah CY, Campbell BA, Seymour JF. Primary breast lymphoma. Cancer Treat Rev 2014;40:900-8.
- Ryan G, Martinelli G, Kuper-Hommel M, Tsang R, Pruneri G, Yuen K, et al. Primary diffuse large B-cell lymphoma of the breast: Prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2008;19:233-41.
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82.
- Jeanneret-Sozzi W, Taghian A, Epelbaum R, Poortmans P, Zwahlen D, Amsler B, et al. Primary breast lymphoma: Patient profile, outcome and prognostic factors. A multicentre Rare Cancer Network study. BMC Cancer 2008;8:86.
- Avilés A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: Results of a controlled clinical trial. Oncology 2005;69:256-60.
- Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: The impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 2010;21:1046-52.
- Guirguis HR, Cheung MC, Mahrous M, Piliotis E, Berinstein N, Imrie KR, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: A single centre experience and review of the literature. Br J Haematol 2012;159:39-49.

| Figure 1:H and E (×40) section showing diffuse proliferation of large cells with high mitotic rate

| Figure 2:Immunohistochemistry stain showing CD20 positivity of diffuse large B-cells

| Figure 3:Computed tomography scan showing a large multi-lobulated soft tissue density lesion involving all quadrants of right breast reaching up to the skin measuring 66 mm × 68 mm × 84 mm

| Figure 4:Interim computed tomography scan done after four cycles of cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab showing marked reduction in size of right breast mass from 6.6 cm × 6.8 cm to 3.4 cm × 1.4 cm
References
- Yoshida S, Nakamura N, Sasaki Y, Yoshida S, Yasuda M, Sagara H, et al. Primary breast diffuse large B-cell lymphoma shows a non-germinal center B-cell phenotype. Mod Pathol 2005;18:398-405.
- Lyons JA, Myles J, Pohlman B, Macklis RM, Crowe J, Crownover RL. Treatment of prognosis of primary breast lymphoma: A review of 13 cases. Am J Clin Oncol 2000;23:334-6.
- Arnold SF, Caron AJ, Peter M, Jon CA. Non-Hodgkin's lymphoma. DeVita, Hellman, and Rosenberg's cancer: Principles & practice of oncology: 10th ed. Wolters Kluwer Health Adis (ESP); 2015. p. 1552-83.
- Yhim HY, Kang HJ, Choi YH, Kim SJ, Kim WS, Chae YS, et al. Clinical outcomes and prognostic factors in patients with breast diffuse large B cell lymphoma: Consortium for Improving Survival of Lymphoma (CISL) study. BMC Cancer 2010;10:321.
- Ganjoo K, Advani R, Mariappan MR, McMillan A, Horning S: Non-Hodgkin lymphoma of the breast. Cancer 2007;110:25-30.
- Cheah CY, Campbell BA, Seymour JF. Primary breast lymphoma. Cancer Treat Rev 2014;40:900-8.
- Ryan G, Martinelli G, Kuper-Hommel M, Tsang R, Pruneri G, Yuen K, et al. Primary diffuse large B-cell lymphoma of the breast: Prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2008;19:233-41.
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82.
- Jeanneret-Sozzi W, Taghian A, Epelbaum R, Poortmans P, Zwahlen D, Amsler B, et al. Primary breast lymphoma: Patient profile, outcome and prognostic factors. A multicentre Rare Cancer Network study. BMC Cancer 2008;8:86.
- Avilés A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: Results of a controlled clinical trial. Oncology 2005;69:256-60.
- Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: The impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 2010;21:1046-52.
- Guirguis HR, Cheung MC, Mahrous M, Piliotis E, Berinstein N, Imrie KR, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: A single centre experience and review of the literature. Br J Haematol 2012;159:39-49.


 PDF
PDF  Views
Views  Share
Share

