Primary anaplastic astrocytoma of the brain after prophylactic cranial irradiation in a case of acute lymphoblastic leukemia: Case report and review of the literature
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(01): 86-88
DOI: DOI: 10.4103/0971-5851.133729
Abstract
A 6½-year-old boy had developed acute lymphoblastic leukemia and was treated with systemic chemotherapy, intrathecal triple drug regimen and prophylactic cranial irradiation. After 10 years he developed anaplastic astrocytoma of the postero-superior cerebellum on the left side while leukemia was in remission. He was treated with surgical excision, followed by adjuvant three dimensional conformal radiotherapy and is on salvage chemotherapy with temozolamide. It is possible that the anaplastic astrocytoma may be a radiation induced malignancy.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
A 6½-year-old boy had developed acute lymphoblastic leukemia and was treated with systemic chemotherapy, intrathecal triple drug regimen and prophylactic cranial irradiation. After 10 years he developed anaplastic astrocytoma of the postero-superior cerebellum on the left side while leukemia was in remission. He was treated with surgical excision, followed by adjuvant three dimensional conformal radiotherapy and is on salvage chemotherapy with temozolamide. It is possible that the anaplastic astrocytoma may be a radiation induced malignancy.
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children. Currently, the overall 5 year relative survival is 80%. Curative therapy includes multi-agent chemotherapy and prophylaxis against central nervous system (CNS) leukemia with intrathecal chemotherapy and/or cranial radiation.
Cranial irradiation has been shown to result in increasing survival of children with ALL.[1] However with increasing survival, there is an increasing risk of secondary brain tumors after cranial irradiation.[2,3,4] Gliomas, meningiomas and sarcomas are the most frequent.[5] We report a case of child in whom anaplastic astrocytoma developed after prophylactic whole brain irradiation for the treatment of ALL.
This was a case report of a 6-year-old boy who was diagnosed with ALL-L2 (French American British Classification) in 2001 and was treated as per multi center protocol (MCP) 843 protocol. Prophylactic irradiation to whole brain with cobalt 60 γ rays to a total dose of 1800 cGy in 10 fractions at 180 cGy/fraction was given from 13th to 23rd September 2001. In December 2003, all medications were stopped after proving complete remission by bone marrow examination.
In April 2011, nearly 10 years post-radiotherapy, he presented with speech difficulty and gait ataxia of 3 months duration. On examination, apart from subnormal intelligence and cerebellar signs he had no other deficits. Magnetic resonance imaging-brain showed – An ill- marginated infiltrating mass lesion in postero-superior cerebellar hemisphere with extension across the midline to involve left cerebellar hemisphere, compressing and displacing the 4th ventricle [Figure 1].
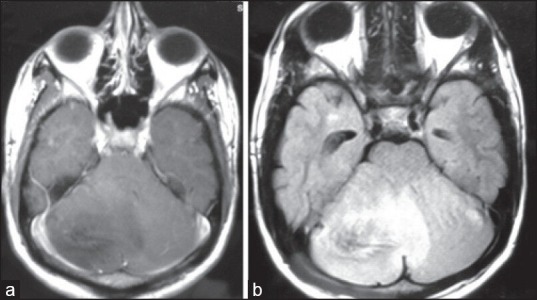
| Figure 1:(a) T1 contrast showing homogenously hypointense lesion with minimal patchy enhancement in cerebellum (b) T2 fluid attenuated inversion recovery showing homogenously hypertintense lesion in cerebellum
He underwent midline sub occipital craniotomy and gross total excision of the tumor. Histopathology examination showed a moderately cellular tumor with a fibrillary background infiltrating into the cerebellar parenchyma [Figure 2a]. The tumor cells displayed moderate nuclear pleomorphism and frequent mitotic figures, including atypical forms [Figure 2b]. Tumor cells were immunopositve for glial fibrillary acidic protein [Figure 2c] and p53. MIB-1 labeling index was approximately 25% in the highest proliferating areas [Figure 2d]. A diagnosis of anaplastic astrocytoma, World Health Organization grade III, was made. Post-operatively he was treated with adjuvant radiation therapy with three dimensional conformal radiotherapy to a dose of 60 GY in 30 fractions at 2 Gy/fraction. Post- radiotherapy, at 6 weeks follow-up, he had residual disease and hence was started on salvage chemotherapy with temozolamide. Presently, patient has only cerebral ataxia and no evidence of ALL.
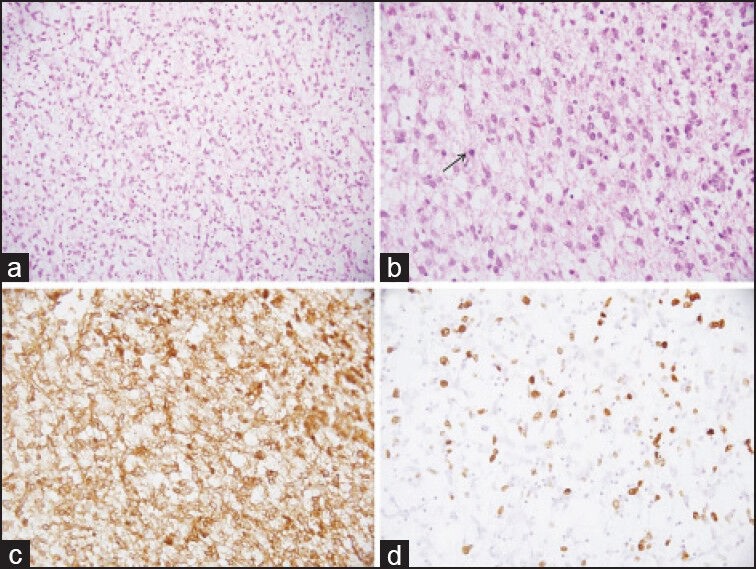
| Figure 2:Photomicrographs of the tumor showing moderate cellularity in a fibrillary background (a, H and E, ×200). The tumor cells show moderate nuclear pleomorphism, frequent mitoses (arrow) (b, H and E, ×400), immunopositivity for glial fibrillary acidic protein (c, immunohistochemical [IHC]; ×400) and high MIB-1 labeling index (d, IHC; ×400)
DISCUSSION
Although most children with ALL become 5 year survivors, second malignancies and other adverse events reduce long-term survival.[3,4,5,6] At 15 year cumulative incidence of second malignancies for ALL survivors is about 2.5-4%. However, with increasing duration of follow-up a cumulative incidence of 6% at 30 years is noted. The most commonly observed secondary malignancies include CNS tumors, leukemias/lymphomas and skin cancers. Risk factors associated with secondary malignancies in ALL survivors include, age at diagnosis (age <5 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4080671/#ref3" rid="ref3" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_387069035" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>3] Gilman and Miller[7] have explained the possible pathogenesis for secondary malignancies, as intrinsic - due to pleiotropic gene action, cytogenetic abnormalities associated with ALL, or immunoregulatory dysfunction; or extrinsic - due to carcinogen that caused ALL, and the treatment administered - as a mere chance of coincidence.
Induction of second malignancies after radiation is well-recognized. This risk was first recognized among children who developed brain tumors after radiation therapy for tinea capitis in the 1940s and 1950s, and was reported among children exposed to as little as 2·5 Gy. Most common second malignancies in CNS include gliomas and meningiomas. The first fully documented case of CNS glioma following prophylactic treatment of ALL was reported in 1979.[8] Similar cases have been reported, which is systematically evaluated by Bowers et al.[9] and concluded that survivors of childhood cancer who received cranial radiation therapy have an increased risk for subsequent CNS neoplasms.
The standardized incidence ratios for subsequent gliomas ranged from 8·9 to 24·3. The interval between first cancer diagnosis and diagnosis of a subsequent high-grade glioma was 8-20 years.[9] There is a linear correlation between the dose of cranial radiation and relative risk of subsequent high-grade gliomas, odds ratios (ORs) for subsequent glioma increased with a dose of radiation exposure and peaked at exposures of 30-44·9 Gy (OR-21).[9]
At 5-year survival for patients with subsequent high-grade gliomas ranged from 0% to 19·5%, which is similar to the 5 year survival rate of 16·6% reported in young adults in the general population.[9]
Well-accepted criteria have been established to show a causal relationship between radiotherapy and occurrence of subsequent neoplasm. These includes prolonged delay between irradiation and detection of a second tumor, second malignancy occurring in the irradiated field, histology of the second tumor differs from the initial neoplasm and the incidence of the second lesion is significantly higher than in controls.[10]
Our case report clearly demonstrates that a possible radiation induced high-grade glioma in the previously irradiated area, as there is in field occurrence of a second type of malignancy, which is histological different from the initial primary leukemic cause, after 10 years of radiation.
In addition this is the first report of a secondary anaplastic astrocytoma developing in the cerebellum in a treated case of ALL.
CONCLUSION
Long-term survivors of patients treated for ALL possibly have increased risk of developing secondary CNS neoplasm especially those who have received cranial irradiation; and that these tumors are of high grade, aggressive clinical behavior and less amenable for treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet 2008;371:1030-43.
- Neglia JP, Meadows AT, Robison LL, Kim TH, Newton WA, Ruymann FB, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med 1991;325:1330-6.
- Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083-95.
- Hope AJ, Mansur DB, Tu PH, Simpson JR. Metachronous secondary atypical meningioma and anaplastic astrocytoma after post-operative craniospinal irradiation for medulloblastoma. Childs Nerv Syst 2006;22:1201-7.
- Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst 2009;101:946-58.
- Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med 2003;349:640-9.
- Gilman PA, Miller RW. Cancer after acute lymphocytic leukemia. Am J Dis Child 1981;135:311-2.
- Walters TR. Childhood acute lymphocytic leukemia with a second primary neoplasm. Am J Pediatr Hematol Oncol 1979;1:285-7.
- Bowers DC, Nathan PC, Constine L, Woodman C, Bhatia S, Keller K, et al. Subsequent neoplasms of the CNS among survivors of childhood cancer: A systematic review. Lancet Oncol 2013;14:e321-8.
- Cahan WG, Woodard HQ. Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1948;1:3-29.

| Figure 1:(a) T1 contrast showing homogenously hypointense lesion with minimal patchy enhancement in cerebellum (b) T2 fluid attenuated inversion recovery showing homogenously hypertintense lesion in cerebellum

| Figure 2:Photomicrographs of the tumor showing moderate cellularity in a fibrillary background (a, H and E, ×200). The tumor cells show moderate nuclear pleomorphism, frequent mitoses (arrow) (b, H and E, ×400), immunopositivity for glial fibrillary acidic protein (c, immunohistochemical [IHC]; ×400) and high MIB-1 labeling index (d, IHC; ×400)
References
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet 2008;371:1030-43.
- Neglia JP, Meadows AT, Robison LL, Kim TH, Newton WA, Ruymann FB, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med 1991;325:1330-6.
- Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083-95.
- Hope AJ, Mansur DB, Tu PH, Simpson JR. Metachronous secondary atypical meningioma and anaplastic astrocytoma after post-operative craniospinal irradiation for medulloblastoma. Childs Nerv Syst 2006;22:1201-7.
- Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst 2009;101:946-58.
- Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med 2003;349:640-9.
- Gilman PA, Miller RW. Cancer after acute lymphocytic leukemia. Am J Dis Child 1981;135:311-2.
- Walters TR. Childhood acute lymphocytic leukemia with a second primary neoplasm. Am J Pediatr Hematol Oncol 1979;1:285-7.
- Bowers DC, Nathan PC, Constine L, Woodman C, Bhatia S, Keller K, et al. Subsequent neoplasms of the CNS among survivors of childhood cancer: A systematic review. Lancet Oncol 2013;14:e321-8.
- Cahan WG, Woodard HQ. Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1948;1:3-29.


 PDF
PDF  Views
Views  Share
Share

