Pregnancy and Acute Lymphoblastic Leukemia: A Case Series and Review of Literature
CC BY 4.0 · Indian J Med Paediatr Oncol 2024; 45(05): 443-450
DOI: DOI: 10.1055/s-0043-1766129
Abstract
Acute lymphoblastic leukemia (ALL) diagnosed during pregnancy is rare and causes ethical and therapeutic challenges. We performed a retrospective search of ALL patients (n = 202) treated at our institution from 2015 to 2020 and found five patients diagnosed during pregnancy. In this report, we discuss the individual patients in detail and the challenges faced during their treatment. The use of established lymphoblastic leukemia treatment protocols and the modifications made therein to prevent untoward chemotherapy-related toxicities to the fetus are discussed in this study. We report the second use of rasburicase during pregnancy in literature with favorable maternal and fetal outcomes. We also present an extensive literature review of 41 cases of ALL in pregnancy previously reported. It is important to note that there is a dearth of guidelines for the treatment of these complex situations, and although certain general principles can be established, an individualized approach is needed in most cases of leukemia diagnosed during pregnancy.
Keywords
acute leukemia - ethical considerations - pregnancy - maternal well-being - fetal outcome - chemotherapy
Authors' Contributions
S.B. was involved in conceptualization, data collection, and manuscript drafting. S.G. helped in conceptualization, clinical data curation, and manuscript editing and analysis. S.S.R., S. Samanta, N.S. and S. Saha were involved in Institutional Medical Boards convened for patient management strategies. M.B. was involved in conceptualization, manuscript editing, analysis, and overall supervision.
Ethics Approval
Retrospective study
Consent for Publication
Yes.
Availability of Data and Material
Available for publication/review.
Publication History
Article published online:
17 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
We recommend
Posterior reversible encephalopathy syndrome in pediatric acute leukemia: Case series and literature review
M Appachu Appachu, Indian Journal of Medical and Paediatric Oncology, 2014
Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of Literature
Abhilasha Sampagar, VCOT Open
Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of Literature
Abhilasha Sampagar, Indian Journal of Medical and Paediatric Oncology
Cesarean Scar Ectopic Pregnancy: Case Series and Review of the Literature
Homayoun Sadeghi, American Journal of Perinatology-1, 2009
Cesarean Scar Ectopic Pregnancy: Case Series and Review of the Literature
Homayoun Sadeghi, American Journal of Perinatology, 2009
Acute leukemia and pregnancy
D. Veneri, Clinical and Experimental Obstetrics & Gynecology, 1996
Colon carcinoma in pregnancy. Case report and review of literature
H. Zakut, Clinical and Experimental Obstetrics & Gynecology, 1984
Subclavian Artery Stenosis: A Case Series and Review of the Literature
Javier Reyna, Reviews in Cardiovascular Medicine, 2014
Uterine rupture in pregnancy: two case reports and review of literature
A. Pontis, Clinical and Experimental Obstetrics & Gynecology, 2016
Adult-onset Still’s disease in pregnancy: case report and literature review
Clinical and Experimental Obstetrics & Gynecology, 2021
Abstract
Acute lymphoblastic leukemia (ALL) diagnosed during pregnancy is rare and causes ethical and therapeutic challenges. We performed a retrospective search of ALL patients (n = 202) treated at our institution from 2015 to 2020 and found five patients diagnosed during pregnancy. In this report, we discuss the individual patients in detail and the challenges faced during their treatment. The use of established lymphoblastic leukemia treatment protocols and the modifications made therein to prevent untoward chemotherapy-related toxicities to the fetus are discussed in this study. We report the second use of rasburicase during pregnancy in literature with favorable maternal and fetal outcomes. We also present an extensive literature review of 41 cases of ALL in pregnancy previously reported. It is important to note that there is a dearth of guidelines for the treatment of these complex situations, and although certain general principles can be established, an individualized approach is needed in most cases of leukemia diagnosed during pregnancy.
Keywords
acute leukemia - ethical considerations - pregnancy - maternal well-being - fetal outcome - chemotherapy
Introduction
Acute leukemia is uncommonly encountered during pregnancy, occurring in approximately 1 in 75,000 cases and its management is a challenging task.[1] It requires a multidisciplinary approach to treat this life-threatening condition, keeping in mind the health and well-being of the mother and fetus. Patient management entails a gamut of challenges from treatment decisions to ethical and social considerations. Moreover, there is a dearth of trials in this unique and rare cohort of patients. Data from case reports, case series, and retrospective studies are the only evidence available, thereby emphasizing the need for an individualized approach.
Herein we report five cases of acute lymphoblastic leukemia (ALL) diagnosed during pregnancy at our center and challenges faced in managing these patients. We also reviewed the available literature to summarize the data on the clinical presentation, treatment complications, maternal, and fetal outcomes of these patients.
This is a series of ALL patients presenting during pregnancy who were treated at our institute between January 2015 and July 2020 (5 years). Clinical and laboratory data and outcomes of these patients were retrieved from our archives and reviewed for the purpose of this report. Informed consent was taken from the patients and/or next of kin while reporting these cases. For each patient, an institutional medical board, comprising specialists from hematology, obstetrics, neonatology, anesthesiology, and transfusion medicine, was convened to formulate the management plan. For the management of ALL in the adolescent and young adults age group, our institutional practice is to use pediatric inspired protocols, namely, the Berlin Frankfurt Munster-2002 regimen. Our institute has a state-of-the art neonatal intensive care unit that can manage preterm births as early as 25 weeks of gestational age.
A total of 202 ALL patients were treated during the period 2015 to 2020 at our institution of which five were ALL with pregnancy (B-ALL, n = 4; Philadelphia positive B-ALL, n = 1). All five cases were women aged between 20 and 29 years (median age: 26 years) of whom three were primigravidae. None of the patients were diagnosed in the first trimester (n = 2, third trimester, n = 3, second trimester). The first two patients involving late third trimester mothers with newly diagnosed ALL were relatively stable and therefore could be managed with transfusion support till delivery, after which standard chemotherapy was administered. In the other three patients, chemotherapy had to be started with the fetus in-utero as a life saving measure for the mother. Institutional protocol for ALL induction therapy comprised of prednisolone at 60 mg/m2, vincristine at 1.4 mg/m2, daunorubicin at 30mg/m2, and L-asparaginase at 5000 IU/m2 in accordance with the BFM-2002 protocol in phase IA and 6-mercaptopurine, cyclophosphamide, and cytarabine in phase IB, followed by consolidation, re-induction and maintenance treatment limbs.
Case Series
Patient 1: A 28-year-old third gravida (32 weeks gestation) who presented with anemia was diagnosed with intermediate-risk Philadelphia negative pre-B-ALL. Her pregnancy was supported till 37 weeks with transfusions after which she delivered a healthy male baby by normal vaginal delivery (NVD). Subsequently BFM-2002 protocol was started and the induction was complicated by recurrent episodes of maxillary sinusitis, sepsis (Klebsiella species), and central line associated blood stream infection, which were managed with appropriate antibiotics. Subsequent phases of chemotherapy were administered without any interruptions.
Patient 2: A 24-year-old primigravida at 35 weeks gestation, developed intermittent fever, and generalized weakness of 1 month duration and was diagnosed as intermediate risk pre-B-ALL. Similar to patient 1, she was also kept on supportive care with packed red blood cells and platelets till she delivered a healthy male child by NVD at 37 weeks gestation. Subsequently, she was initiated on BFM-2002 protocol and received chemotherapy without any modifications.
Patient 3: A 29-year-old primigravida at 28 weeks of gestation was diagnosed as Philadelphia negative pre-B-ALL with leucocytosis (50,000/cumm with 80% blasts) and retinal hemorrhage. She received BFM-2002 induction without any modifications. Fetal assessment during leukemia induction revealed no anomalies. At 35 weeks of gestation and at the end of induction IA when her counts had recovered, she underwent planned Cesarean Section (CS) and delivered a healthy baby boy, appropriate for gestational age. Subsequently she received phase IB of BFM-2002. She unfortunately relapsed post-induction, and therapy was switched to rituximab-hyper-CVAD (comprising of hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone). During the second cycle of hyper-CVAD, she developed febrile neutropenia, septic shock, and succumbed to it. The child was healthy at last known follow-up.
Patient 4: A 26-year-old third gravida (26 weeks) presented with fever, generalized lymphadenopathy, and multiple skin nodules and plaques. On evaluation, she had hyperleukocytosis (with 90% blasts), anemia, and thrombocytopenia and was diagnosed as Philadelphia positive (Ph + ) B-ALL with leukemia cutis and central nervous system (CNS) involvement. She developed tumor lysis syndrome (TLS) and was given rasburicase during initial stabilization. She was then started on modified E-WALL protocol which included a tyrosine kinase inhibitor (TKI)—imatinib 600 mg daily along with weekly pulsed dexamethasone and vincristine injections. Intrathecal chemotherapy was given with cytarabine and hydrocortisone, while methotrexate was omitted. Her platelet counts recovered by 31st week of gestation. Her pregnancy was continued till 32 weeks of gestation after which she underwent an elective CS and gave birth to a healthy female child. Post-induction bone marrow on day 52 of E-WALL protocol was in morphological remission and BCR-ABL measurable residual disease (MRD) was negative. She received one cycle of E-WALL consolidation after which she was lost to follow-up.
Patient 5: The last case is that of a 20-year-old primigravida at 24 weeks gestation who presented with progressive weakness, exertional dyspnea, low-grade fever, and was found to have severe anemia and atypical cells in peripheral blood. A diagnosis of Ph negative pre-B-ALL was made. She developed hepatic encephalopathy, TLS, and septic shock that were managed effectively. In this background of deranged liver functions and an early pregnancy, she was started on a modified BFM-2002 protocol, consisting of steroids and vincristine only. Subsequently, she went on to receive a modified phase IB of BFM-2002 protocol where only the cytarabine blocks were administered intravenously and intrathecal cytarabine chemotherapy was given, while omitting methotrexate, 6-mercaptopurine, and cyclophosphamide from the protocol. Her phase IB of induction was completed at 32 weeks of gestation. Pregnancy continued till term and she delivered a healthy male child. Post-induction, bone marrow was in remission and MRD was negative. However, she had persistence of CNS disease and was given high-dose intravenous methotrexate consolidation @ 5gm/m2 along with triple intrathecal chemotherapy. Her reinduction chemotherapy phase was interrupted by coronavirus disease 2019 pandemic. Subsequently on resumption of chemotherapy, she developed febrile neutropenia, macrophage activation syndrome, went into septic shock, and unfortunately succumbed to her illness.
A summary of the patients, course of treatment in hospital, and their outcome are mentioned in [Table 1].
|
Case no. |
Patient details (age/obstetric history/POG) |
Presenting counts (Hb [g/dl]/total leucocyte count (cumm)/platelets (cumm)/blast [%]) |
Diagnosis IPT/CNS status/Ph status/CG/risk group/EM disease |
Protocol |
Indication for treatment; modification done |
Significant Issues faced during treatment |
Disease outcome |
Pregnancy outcome |
Last follow-up |
|---|---|---|---|---|---|---|---|---|---|
|
1 |
28 y G3P2 32 weeks |
9.1/41,500/20,000/ 50% blasts |
Pre-B-ALL/CNS-1/ Ph- neg/46XX/IRG |
BFM-2002 |
Protocol started post-delivery No dose adjustments |
Induction: Maxillary sinusitis, CLABSI and sepsis |
Remission |
NVD, male baby@37 weeks gestation |
4 years: mother and child doing well |
|
2 |
24 y G1P0 35 weeks |
9.4/10600/218,000/ 30% blasts |
Pre-B-ALL/ CNS-1/ Ph- neg/ 46XX/ IRG |
BFM-2002 |
Protocol started post-delivery Vinblastine given in view of peripheral neuropathy |
Induction- CLABSI, genital herpes, pneumonia, vincristine induced PN |
Remission |
NVD, male baby@ 37 weeks gestation |
3 years: mother and child doing well |
|
3 |
29 y G1P0 28 weeks, 4 days |
8.3/50000/20000/ 80% blasts |
Pre B-ALL/CNS-1/Ph- neg/NDC/IRG |
BFM-2002 |
Protocol started in third trimester in view of bleeding. No dose modification. Phase IB given post-delivery |
Induction- cytopenia Relapsed prior to consolidation. Switched to Hyper-CVAD. |
Relapse post-induction 2# Hyper-CVAD: death due to septic shock |
Elective CS Male baby @ 35 weeks |
Child was healthy at last follow-up |
|
4 |
26y G3P2 26 weeks |
7/250,000/10,000/ 90% blasts |
Philadelphia +ve B-ALL/CNS-3/46XX/HRG/ leukemia cutis |
Modified E-WALL; Imatinib 600 mg OD |
Induction started in late second trimester. Rasburicase given IT: ARA-C and hydrocortisone. MTX omitted |
TLS |
Remission |
Elective CS, female baby@ 32 weeks |
Lost to follow-up after first cycle of consolidation |
|
5 |
20y G1P0 24 weeks |
6/84,600/25,000/ 40% blasts |
Pre-B-ALL/CNS-1/BCR- ABL neg/46XX/IRG |
BFM 2002 with changes |
Induction: second trimester: VCR and steroid only IB: Only Ara-C; IT ARA-C Consolidation: MRC UK-ALL HD-MTX @ 5gm/m2 and L-ASP 10000 IU/m2 triple IT with MTX, ARA-C and hydrocortisone |
Pre-treatment: Hepatic encephalopathy, septic shock, TLS Consolidation: CNS relapse Reinduction: therapy interruption due to COVID-19 pandemic |
Remission post-induction Consolidation: Isolated CNS relapse Re-induction: Death due to septic shock and MAS |
Elective CS, male baby@ 37 weeks |
Child healthy at the time of her death |
|
Sl. no. |
Reference (total cases; ALL cases) |
Patient details (age/obstetric history/POG) |
Diagnosis |
Therapy used; modifications (if any) |
Pregnancy and fetal outcome |
Disease status |
Maternal outcome |
|---|---|---|---|---|---|---|---|
|
1 |
Krueger et al [1976] (4) |
15/-/26 |
ALL |
COAP regimen: cyclophosphamide, vincristine, cytarabine, prednisone |
Induction of labor. Normal Infant at 38 weeks |
PD |
Relapse1-month post-partum |
|
2 |
O' Donnell et al [1979] (4) |
24/-/15 |
ALL |
TAD regimen (thioguanine, cytarabine, daunorubicin) |
Pre-eclampsia and intrauterine fetal death at 30 weeks |
CR |
Alive |
|
3 |
Okun et al [1979] (5) |
18/-/12 |
ALL |
1st Induction: VCR, Pred, IT MTX. 2nd induction: CTX, L-Asp, DNR, 6-mercaptopurine; WBRT |
CS at 31 weeks; baby with transient pancytopenia, CHF, normal development at 1 year |
Relapse |
CNS relapse 5 weeks post-partum |
|
4 |
Dara et al [1981] (4) |
26/-/21 |
ALL |
6-MP, MTX, discontinued when pregnancy confirmed; relapse at 21 weeks, second line initiated with doxorubicin, VCR, Pred, Cyt, MTX |
CS at 36 weeks. infant with polycythemia and hyperbilirubinemia Normal growth and development at 6 months |
CR |
Alive |
|
5 |
Sigler et al [1988] (4) |
26/-/32 |
ALL |
Pre, DNR, Ctx, Cyt, L-asp |
Induction at 35 weeks. Normal infant |
CR |
Remission followed by maintenance |
|
6 |
Avasthi et al [1993] (4) |
20/-/22 |
ALL |
Two courses of VCR, Pred |
Preterm delivery at 29 weeks to live infant |
– |
Sudden death 2 days after delivery |
|
7 |
Camera et al [1996] (4) |
21/-/17 |
ALL |
VCR, Pred, DNR, L-asp |
C-section at 29 weeks Normal male infant |
Relapse |
Death 9 months later from relapse |
|
8 |
Tewari et al [1999] (4) |
17/-/33 |
ALL |
VCR, Pred for relapse ALL |
Induction at 35 weeks Normal infant |
CR |
Consolidation s/p allo- SCT 22 months later |
|
9 |
Hansen et al [2001] (4) |
24/-/26 |
ALL |
Induction CALGB 9111. At 26 weeks: DNR, VCR, Pred, L-asp At 30 & 34 weeks: IT-MTX, Ctx,6-MP, Cyt, VCR, L-asp |
Spontaneous delivery at 36 weeks. Normal male infant |
– |
Unclear |
|
10 |
Ali et al [2002] (10 cases: 2-ALL) (5) |
24/G1/24 21/G2P0/8 |
B-ALL Relapsed B-ALL |
Not documented Not documented |
Therapeutic abortion Therapeutic abortion |
Remission relapse |
Alive Dead |
|
11 |
Terek et al 2003] (4) |
21/-/31 |
ALL |
VCR, DNR, Pred, L-asp |
C-section, newborn respiratory distress (required intubation) |
– |
Maternal death due to sepsis |
|
12 |
Chelghoum et al [2005] (n = 37) (6) 6 ALL cases |
1. 25/G1/27 2. 34/G2/9 3. 33/G4/26 4. 30/G1/10 5. 21/G1/28 6. 25/G1/9 |
T-ALL Pre B-ALL Pre B-ALL Ph+ B-ALL Pre B-ALL T-ALL |
All cases received VCR + Dauno + CTX + Pred |
NVD, premature Therapeutic abortion CS; premature Therapeutic abortion CS; premature Therapeutic abortion |
PD CR CR CR CR CR |
|
|
13 |
Molkenboer et al [2005] 2 ALL cases (7) |
1. 30/G3P2/6 2. 37/G1/15 |
Ph+ B-ALL Ph+ B-ALL |
Pred + VCR + Dauno + Asp + ITMTx; High-dose cytarabine+ imatinib Same as above |
Missed abortion at 11 weeks Spontaneous delivery at 22 weeks; stillborn |
CR PD post-induction |
Death post-HSCT Imatinib palliation. Death few weeks later |
|
14 |
Dilek et al [2006] (1/21, ALL) (8) |
25/G1/term |
ALL |
4 drug regimen induction |
NVD; LBW |
CR post-induction |
Alive |
|
15 |
Matsouka et al [2007] (9) |
16/G1/26 + 3d |
B-ALL |
BFM-95; Recombinant G-CSF use during cytopenic phase. Delivered post-induction |
Elective CS at 32.4 weeks; LBW |
CR |
Alive |
|
16 |
Papantoniou et al [2008] (4) |
16/-/26 |
ALL |
DNR, VCR, L-asp, Pred, IT-Mtx, G-CSF |
CS at 32 weeks. Infant normal at 18 months |
CR |
Remission at 18-months follow-up |
|
17 |
Udink Ten Cate et al [2009] (4) |
30/-/23 |
ALL |
VCR, Pre, IT-MTX, Ctx, DNR. VCR, Ctx, DNR. Maintenance 6-MP |
PROM at 33 weeks, NVD baby; pancytopenic, normal development at 2 years |
CR |
MUD—HSCT In CR 2 years after transplant |
|
18 |
Aljurf et al [2009] 2 ALL cases (4) |
37/-/29 27/-/13 |
ALL ALL |
VCR, dexamethasone, idarubicin Unknown |
Full-term infant; anemia Spontaneous abortion at 14 weeks during induction |
CR CR |
CR with induction Allo-SCT in CR 1 Alive 4 years later |
|
19 |
Ticku et al [2013] (4) |
22/G1/26 |
Ph+ B-ALL |
Induction: Hyper-CVAD + dasatinib Ph+ mutation F317L; Ponatinib started 10 days post-partum |
Elective CS at 30 weeks; LBW |
CR |
Alive |
|
20 |
Nakajima et al [2013] (10) 3 ALL cases |
1. 20/G1/37 2. 36/G1/29 3. 29/G1/5 |
ALL ALL/ t(9;22) ALL |
Not available DNR + VCR + , CTX + Pred Not available |
Emergency CS; live birth Elective CS; live birth Therapeutic abortion |
PD CR CR |
Dead Alive Alive |
|
21 |
Saleh et al [2014] (n = 32; 6 ALL cases) (2) |
1. 23/G2/38 2. 25/G1/12 3. 26/G3/28 4. 23/G3/13 5. 37/G7/29 6. 21/G1/31 |
Pre B-ALL Pre B ALL Pre B-ALL Pre B-ALL Pre B- ALL T-ALL |
None None 5 drug regime None 5 drug regime VCR + Pred |
Live birth at term Spontaneous abortion Spontaneous abortion Spontaneous abortion Live birth at term Preterm birth at 33+ weeks |
PD PD CR PD LTFU PD |
Death Death Alive, post-SCT Dead, post-SCT LTFU Death |
|
22 |
Farhadfar et al [2016] (n = 23) 5 ALL cases (11) |
1. 26/-/12 2. 23/-/6 3. 34/-/10 4. 19/-/16 5. 28/-/35 |
B-ALL B-ALL Ph+ ALL B-ALL B-ALL |
Post-termination induction C10403 Post-termination induction C10403 CALGB9111; Reinduction: Imatinib with ara-C HSCT: MUD, TBI/VP-16 DNR/VCR/Pred; Consolidation: HiDAC Post-delivery - DNR/VCR/Pred Relapse: DNR/VCR/Pred, HiDAC, MTX/L-Asp |
Therapeutic abortion Therapeutic abortion Fetal loss at 19 weeks Fetal loss 22 weeks NVD,38 weeks |
- CR CR, f/b HSCT CR PD |
Alive Alive Death on D21 of HSCT d/t septic shock Death Death |
|
23 |
Vlijm-Kievit et al [2017] (12) |
37/G2P1/36 |
T-ALL |
Pred + VCR + Dauno + Peg-asparaginase (HOVON 100 protocol)- Asp and MTX delayed till post-delivery Therapeutic LMWH given upto 6 weeks post-partum |
NVD 37 weeks |
Remission |
Alive |
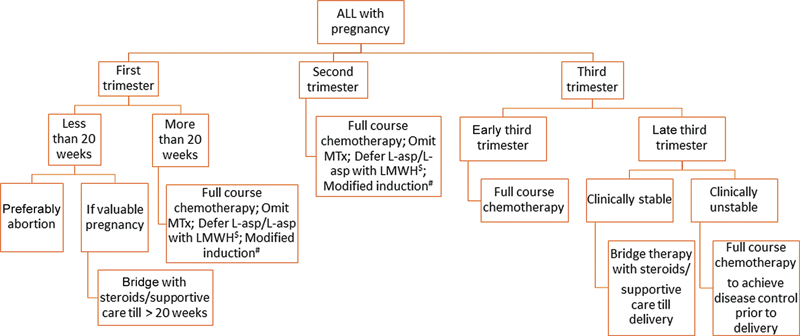
Fig. 1 Flowchart of management of acute lymphoblastic leukemia in pregnancy. $: L-asparaginase is associated with increased risk of thrombosis during pregnancy and should be combined with low molecular weight heparin (LMWH) prophylaxis or deferred till after delivery.#: Modification of protocol on a case-to-case basis; modification based on co-morbidities/intolerance to specific chemotherapeutics.
Conclusion
Management of leukemia in pregnancy is a challenging task and relies heavily on effort of a multidisciplinary team, from treating hematologists to obstetricians. Individualized approach to manage these patients is essential, considering the gestational timing of presentation of ALL, cytogenetics, clinical profile, and active medical issues at diagnosis. Novel approaches used in our patients such as the use of modified BFM and E-WALL protocols, modifications with regard to timing of L-asparaginase administration, and antepartum use of rasburicase were met with favorable pregnancy outcomes.
Conflict of interest
None declared.
Authors' Contributions
S.B. was involved in conceptualization, data collection, and manuscript drafting. S.G. helped in conceptualization, clinical data curation, and manuscript editing and analysis. S.S.R., S. Samanta, N.S. and S. Saha were involved in Institutional Medical Boards convened for patient management strategies. M.B. was involved in conceptualization, manuscript editing, analysis, and overall supervision.
Ethics Approval
Retrospective study
References
- Acute leukemia in pregnancy with ovarian metastasis: a case report and review of the literature - PubMed [Internet]. [cited 2020 Sep 8]. Accessed February 21, 2023 at: https://pubmed.ncbi.nlm.nih.gov/14675333/
- Saleh AJM, Alhejazi A, Ahmed SO. et al. Leukemia during pregnancy: long term follow up of 32 cases from a single institution. Hematol Oncol Stem Cell Ther 2014; 7 (02) 63-68
- Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol 2004; 5 (05) 283-291
- Ticku J, Oberoi S, Friend S, Busowski J, Langenstroer M, Baidas S. Acute lymphoblastic leukemia in pregnancy: a case report with literature review. Ther Adv Hematol 2013; 4 (05) 313-319
- Ali R, Ozkalemkaş F, Ozçelik T. et al. Maternal and fetal outcomes in pregnancy complicated with acute leukemia: a single institutional experience with 10 pregnancies at 16 years. Leuk Res 2003; 27 (05) 381-385
- Chelghoum Y, Vey N, Raffoux E. et al. Acute leukemia during pregnancy: a report on 37 patients and a review of the literature. Cancer 2005; 104 (01) 110-117
- Molkenboer JFM, Vos AH, Schouten HC, Vos MC. Acute lymphoblastic leukaemia in pregnancy. Neth J Med 2005; 63 (09) 361-363
- Dilek I, Topcu N, Demir C. et al. Hematological malignancy and pregnancy: a single-institution experience of 21 cases. Clin Lab Haematol 2006; 28 (03) 170-176
- Matsouka C, Marinopoulos S, Barbaroussi D, Antsaklis A. Acute lymphoblastic leukemia during gestation. Med Oncol 2008; 25 (02) 190-193
- Nakajima Y, Hattori Y, Ito S. et al. Acute leukemia during pregnancy: an investigative survey of the past 11 years. Int J Lab Hematol 2015; 37 (02) 174-180
- Farhadfar N, Cerquozzi S, Hessenauer MR. et al. Acute leukemia in pregnancy: a single institution experience with 23 patients. Leuk Lymphoma 2017; 58 (05) 1052-1060
- Vlijm-Kievit A, Jorna NGE, Moll E. et al. Acute lymphoblastic leukemia during the third trimester of pregnancy. Leuk Lymphoma 2018; 59 (05) 1274-1276
- Santiago-López CJ, Cuan-Baltazar Y, Pérez-Partida AM, Muñoz-Pérez MJ, Soto-Vega E. Leukemia during pregnancy. Obstet Gynecol Int J 2017; 6 (06) 00225
- Kauss MA, Reiterer G, Bunaciu RP, Yen A. Human myeloblastic leukemia cells (HL-60) express a membrane receptor for estrogen that signals and modulates retinoic acid-induced cell differentiation. Exp Cell Res 2008; 314 (16) 2999-3006 https://pubmed.ncbi.nlm.nih.gov/18692045/ cited 2020Oct23 [Internet]
- Milojkovic D, Apperley JF. How I treat leukemia during pregnancy. Blood 2014; 123 (07) 974-984
- Brenner B, Avivi I, Lishner M. Haematological cancers in pregnancy. Vol. 379, The Lancet. Lancet Publishing Group; 2012: 580-7
- Yarbro CH, Wujcik D, Gobel BH. Cancer Nursing. Jones & Bartlett Learning; 2016. Available at: https://books.google.co.in/books?id=mGt7jgEACAAJ
- Zaidi A, Johnson L-M, Church CL. et al. Management of concurrent pregnancy and acute lymphoblastic malignancy in teenaged patients: two illustrative cases and review of the literature. J Adolesc Young Adult Oncol 2014; 3 (04) 160-175
- Rousselot P, Coudé MM, Gokbuget N. et al; European Working Group on Adult ALL (EWALL) group. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood 2016; 128 (06) 774-782
- Gökbuget N. Treatment of older patients with acute lymphoblastic leukemia. Hematology (Am Soc Hematol Educ Program) 2016; 2016 (01) 573-579
- Pye SM, Cortes J, Ault P. et al. The effects of imatinib on pregnancy outcome. Blood 2008; 111 (12) 5505-5508
- Mukhopadhyay A, Dasgupta S, Kanti Ray U, Gharami F, Bose CK, Mukhopadhyay S. Pregnancy outcome in chronic myeloid leukemia patients on imatinib therapy. Ir J Med Sci 2015; 184 (01) 183-188
- Cole S, Kantarjian H, Ault P, Cortés JE. Successful completion of pregnancy in a patient with chronic myeloid leukemia without active intervention: a case report and review of the literature. Clin Lymphoma Myeloma 2009; 9 (04) 324-327
- Conchon M, Sanabani SS, Serpa M. et al. Successful pregnancy and delivery in a patient with chronic myeloid leukemia while on dasatinib therapy. Adv Hematol 2010; 2010: 136252-136252
- Conchon M, Sanabani SS, Bendit I, Santos FM, Serpa M, Dorliac-Llacer PE. Two successful pregnancies in a woman with chronic myeloid leukemia exposed to nilotinib during the first trimester of her second pregnancy: case study. J Hematol Oncol 2009; 2 (01) 42
- Stary J, Zimmermann M, Campbell M. et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol 2014; 32 (03) 174-184
- Rowe JM, Buck G, Burnett AK. et al; ECOG, MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106 (12) 3760-3767
- Gilis L, Lebras L, Bouafia-Sauvy F. et al. Sequential combination of high dose methotrexate and L-asparaginase followed by allogeneic transplant: a first-line strategy for CD4+/CD56+ hematodermic neoplasm. Leuk Lymphoma 2012; 53 (08) 1633-1637
- Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice. Wolters Kluwer Health; 2011. Available at: https://books.google.co.in/books?id=0U4aj4GZWCIC
- Fernández Fernández C, Pérez Prieto B, Argüelles Álvarez S, García González C, González García C. Leucemia aguda mieloblástica en gestante de 28 semanas. [Acute myeloblastic leukemia in a 28-week pregnant woman] Clin Invest Ginecol Obstet 2008; 35 (05) 184-186
- Shapira T, Pereg D, Lishner M. How I treat acute and chronic leukemia in pregnancy. Blood Rev 2008; 22 (05) 247-259
- Middeke JM, Bruck N, Parmentier S, Bornhäuser M, Schetelig J. Use of rasburicase in a pregnant woman with acute lymphoblastic leukaemia and imminent tumour lysis syndrome. Ann Hematol 2014; 93 (03) 531-532
- Howard SC, Jones DP, Pui C-H. The tumor lysis syndrome. N Engl J Med 2011; 364 (19) 1844-1854
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2011. Available at: https://books.google.co.id/books?id=OIgTE4aynrMC
- Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood 2003; 102 (13) 4306-4311
- Provan D, Stasi R, Newland AC. et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010; 115 (02) 168-186
Address for correspondence
Publication History
Article published online:
17 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
We recommend
Posterior reversible encephalopathy syndrome in pediatric acute leukemia: Case series and literature review
M Appachu Appachu, Indian Journal of Medical and Paediatric Oncology, 2014
Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of Literature
Abhilasha Sampagar, VCOT Open
Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of Literature
Abhilasha Sampagar, Indian Journal of Medical and Paediatric Oncology
Cesarean Scar Ectopic Pregnancy: Case Series and Review of the Literature
Homayoun Sadeghi, American Journal of Perinatology-1, 2009
Cesarean Scar Ectopic Pregnancy: Case Series and Review of the Literature
Homayoun Sadeghi, American Journal of Perinatology, 2009
ENDOCRINOLOGY IN PREGNANCY: Pheochromocytoma in pregnancy: case series and review of literature
K van der Weerd, Political Science Quarterly, 2017
Paraganglioma in Pregnancy: A Case Series and Review of the Literature
Wing, The Journal of Clinical Endocrinology & Metabolism, 2015
Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature
Pasquale Pagliano, Journal of Antimicrobial Chemotherapy, 2005
Granular Acute Lymphoblastic Leukemia
Preston Stein, American Journal of Clinical Pathology, 1983
Childhood Acute Lymphoblastic Leukemia
Jeffrey E. Rubnitz, The Oncologist, 1997

Fig. 1 Flowchart of management of acute lymphoblastic leukemia in pregnancy. $: L-asparaginase is associated with increased risk of thrombosis during pregnancy and should be combined with low molecular weight heparin (LMWH) prophylaxis or deferred till after delivery.#: Modification of protocol on a case-to-case basis; modification based on co-morbidities/intolerance to specific chemotherapeutics.
References
- Acute leukemia in pregnancy with ovarian metastasis: a case report and review of the literature - PubMed [Internet]. [cited 2020 Sep 8]. Accessed February 21, 2023 at: https://pubmed.ncbi.nlm.nih.gov/14675333/
- Saleh AJM, Alhejazi A, Ahmed SO. et al. Leukemia during pregnancy: long term follow up of 32 cases from a single institution. Hematol Oncol Stem Cell Ther 2014; 7 (02) 63-68
- Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol 2004; 5 (05) 283-291
- Ticku J, Oberoi S, Friend S, Busowski J, Langenstroer M, Baidas S. Acute lymphoblastic leukemia in pregnancy: a case report with literature review. Ther Adv Hematol 2013; 4 (05) 313-319
- Ali R, Ozkalemkaş F, Ozçelik T. et al. Maternal and fetal outcomes in pregnancy complicated with acute leukemia: a single institutional experience with 10 pregnancies at 16 years. Leuk Res 2003; 27 (05) 381-385
- Chelghoum Y, Vey N, Raffoux E. et al. Acute leukemia during pregnancy: a report on 37 patients and a review of the literature. Cancer 2005; 104 (01) 110-117
- Molkenboer JFM, Vos AH, Schouten HC, Vos MC. Acute lymphoblastic leukaemia in pregnancy. Neth J Med 2005; 63 (09) 361-363
- Dilek I, Topcu N, Demir C. et al. Hematological malignancy and pregnancy: a single-institution experience of 21 cases. Clin Lab Haematol 2006; 28 (03) 170-176
- Matsouka C, Marinopoulos S, Barbaroussi D, Antsaklis A. Acute lymphoblastic leukemia during gestation. Med Oncol 2008; 25 (02) 190-193
- Nakajima Y, Hattori Y, Ito S. et al. Acute leukemia during pregnancy: an investigative survey of the past 11 years. Int J Lab Hematol 2015; 37 (02) 174-180
- Farhadfar N, Cerquozzi S, Hessenauer MR. et al. Acute leukemia in pregnancy: a single institution experience with 23 patients. Leuk Lymphoma 2017; 58 (05) 1052-1060
- Vlijm-Kievit A, Jorna NGE, Moll E. et al. Acute lymphoblastic leukemia during the third trimester of pregnancy. Leuk Lymphoma 2018; 59 (05) 1274-1276
- Santiago-López CJ, Cuan-Baltazar Y, Pérez-Partida AM, Muñoz-Pérez MJ, Soto-Vega E. Leukemia during pregnancy. Obstet Gynecol Int J 2017; 6 (06) 00225
- Kauss MA, Reiterer G, Bunaciu RP, Yen A. Human myeloblastic leukemia cells (HL-60) express a membrane receptor for estrogen that signals and modulates retinoic acid-induced cell differentiation. Exp Cell Res 2008; 314 (16) 2999-3006 https://pubmed.ncbi.nlm.nih.gov/18692045/ cited 2020Oct23 [Internet]
- Milojkovic D, Apperley JF. How I treat leukemia during pregnancy. Blood 2014; 123 (07) 974-984
- Brenner B, Avivi I, Lishner M. Haematological cancers in pregnancy. Vol. 379, The Lancet. Lancet Publishing Group; 2012: 580-7
- Yarbro CH, Wujcik D, Gobel BH. Cancer Nursing. Jones & Bartlett Learning; 2016. Available at: https://books.google.co.in/books?id=mGt7jgEACAAJ
- Zaidi A, Johnson L-M, Church CL. et al. Management of concurrent pregnancy and acute lymphoblastic malignancy in teenaged patients: two illustrative cases and review of the literature. J Adolesc Young Adult Oncol 2014; 3 (04) 160-175
- Rousselot P, Coudé MM, Gokbuget N. et al; European Working Group on Adult ALL (EWALL) group. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood 2016; 128 (06) 774-782
- Gökbuget N. Treatment of older patients with acute lymphoblastic leukemia. Hematology (Am Soc Hematol Educ Program) 2016; 2016 (01) 573-579
- Pye SM, Cortes J, Ault P. et al. The effects of imatinib on pregnancy outcome. Blood 2008; 111 (12) 5505-5508
- Mukhopadhyay A, Dasgupta S, Kanti Ray U, Gharami F, Bose CK, Mukhopadhyay S. Pregnancy outcome in chronic myeloid leukemia patients on imatinib therapy. Ir J Med Sci 2015; 184 (01) 183-188
- Cole S, Kantarjian H, Ault P, Cortés JE. Successful completion of pregnancy in a patient with chronic myeloid leukemia without active intervention: a case report and review of the literature. Clin Lymphoma Myeloma 2009; 9 (04) 324-327
- Conchon M, Sanabani SS, Serpa M. et al. Successful pregnancy and delivery in a patient with chronic myeloid leukemia while on dasatinib therapy. Adv Hematol 2010; 2010: 136252-136252
- Conchon M, Sanabani SS, Bendit I, Santos FM, Serpa M, Dorliac-Llacer PE. Two successful pregnancies in a woman with chronic myeloid leukemia exposed to nilotinib during the first trimester of her second pregnancy: case study. J Hematol Oncol 2009; 2 (01) 42
- Stary J, Zimmermann M, Campbell M. et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol 2014; 32 (03) 174-184
- Rowe JM, Buck G, Burnett AK. et al; ECOG, MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106 (12) 3760-3767
- Gilis L, Lebras L, Bouafia-Sauvy F. et al. Sequential combination of high dose methotrexate and L-asparaginase followed by allogeneic transplant: a first-line strategy for CD4+/CD56+ hematodermic neoplasm. Leuk Lymphoma 2012; 53 (08) 1633-1637
- Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy: Principles and Practice. Wolters Kluwer Health; 2011. Available at: https://books.google.co.in/books?id=0U4aj4GZWCIC
- Fernández Fernández C, Pérez Prieto B, Argüelles Álvarez S, García González C, González García C. Leucemia aguda mieloblástica en gestante de 28 semanas. [Acute myeloblastic leukemia in a 28-week pregnant woman] Clin Invest Ginecol Obstet 2008; 35 (05) 184-186
- Shapira T, Pereg D, Lishner M. How I treat acute and chronic leukemia in pregnancy. Blood Rev 2008; 22 (05) 247-259
- Middeke JM, Bruck N, Parmentier S, Bornhäuser M, Schetelig J. Use of rasburicase in a pregnant woman with acute lymphoblastic leukaemia and imminent tumour lysis syndrome. Ann Hematol 2014; 93 (03) 531-532
- Howard SC, Jones DP, Pui C-H. The tumor lysis syndrome. N Engl J Med 2011; 364 (19) 1844-1854
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2011. Available at: https://books.google.co.id/books?id=OIgTE4aynrMC
- Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood 2003; 102 (13) 4306-4311
- Provan D, Stasi R, Newland AC. et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010; 115 (02) 168-186


 PDF
PDF  Views
Views  Share
Share

