Poor Risk Advanced Renal Cell Carcinoma: Outcomes from a Registry in a Tertiary Cancer Center
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 311-315
DOI: DOI: 10.4103/ijmpo.ijmpo_154_16
Abstract
Background: Poor-risk advanced Renal cell carcinoma (RCC) are an under-evaluated and difficult to treat subset of patients with poor prognosis. While Temsirolimus is the approved first line therapy for this category, Tyrosine kinase inhibitors (TKIs) are also commonly uses as initial treatment. We present an analysis of poor-risk advanced RCC treated in our institute. Materials and Methods: Patients diagnosed as poor-risk (as per Heng criteria) advanced RCC from June 2008 to December 2015 were analysed for baseline demographics, treatment received, toxicity (primarily Grade 3 and Grade 4), response rates (RR) and survival. Results: 60 patients (43 males, 17 females) with a median age of 53 years were included for final analysis. Median ECOG PS was 1, clear cell was the predominant histology (63.3%), and 46.7% of patients had greater than 2 sites of metastases. Sorafenib, Sunitinib, Temsirolimus and Pazopanib were used to treat 43.3%, 36.7%, 8.3% and 6.7% of patients respectively, while 3 patients were offered upfront best supportive care. Common adverse events included skin rash (31.5%), HFS (Grade 2 and 3 - 30.8%), mucositis (26.3%), hypertension (24.5%), and dyslipidaemias (22.8%). 41 patients were available for response - overall response rate observed was 15%, while clinical benefit rate was 50%. Median progression free survival was 5.78 months (4.67-6.89) and median overall survival (OS) was 10.05 months (7.31-12.79). Conclusion: A majority of poor-risk metastatic RCC patients in our study were treated with TKIs and the survival outcomes appear to suggest that this strategy is a feasible alternative to Temsirolimus in the Indian setting.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Poor-risk advanced Renal cell carcinoma (RCC) are an under-evaluated and difficult to treat subset of patients with poor prognosis. While Temsirolimus is the approved first line therapy for this category, Tyrosine kinase inhibitors (TKIs) are also commonly uses as initial treatment. We present an analysis of poor-risk advanced RCC treated in our institute.
Materials and Methods:
Patients diagnosed as poor-risk (as per Heng criteria) advanced RCC from June 2008 to December 2015 were analysed for baseline demographics, treatment received, toxicity (primarily Grade 3 and Grade 4), response rates (RR) and survival.
Results:
60 patients (43 males, 17 females) with a median age of 53 years were included for final analysis. Median ECOG PS was 1, clear cell was the predominant histology (63.3%), and 46.7% of patients had greater than 2 sites of metastases. Sorafenib, Sunitinib, Temsirolimus and Pazopanib were used to treat 43.3%, 36.7%, 8.3% and 6.7% of patients respectively, while 3 patients were offered upfront best supportive care. Common adverse events included skin rash (31.5%), HFS (Grade 2 and 3 – 30.8%), mucositis (26.3%), hypertension (24.5%), and dyslipidaemias (22.8%). 41 patients were available for response – overall response rate observed was 15%, while clinical benefit rate was 50%. Median progression free survival was 5.78 months (4.67-6.89) and median overall survival (OS) was 10.05 months (7.31-12.79).
Conclusion:
A majority of poor-risk metastatic RCC patients in our study were treated with TKIs and the survival outcomes appear to suggest that this strategy is a feasible alternative to Temsirolimus in the Indian setting.
Introduction
The introduction of targeted agents has transformed the treatment landscape of patient with advanced or metastatic renal cell carcinoma (mRCC). Drugs targeting the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) axis and the mammalian target of rapamycin (mTOR) pathway are now standard options for patients with mRCC.
However, patients with “poor-risk” or “high-risk” features continue to remain an under-evaluated and difficult to treat population, despite comprising 30% of an mRCC population.[1] There also remains some ambiguity as to how to correctly identify poor risk patients, due to the differing criteria used by commonly used risk assessment models such as the Memorial Sloan Kettering Cancer Center (MSKCC) or Heng criteria.[2,3] The advanced renal cell carcinoma (ARCC) trial with temsirolimus assessed only poor-risk patients (albeit by criteria different to MSKCC or Heng) and this is currently the basis for temsirolimus being the recommended first-line option for these patients.[4,5]
While temsirolimus is the recommended option, the tyrosine kinase inhibitors (TKIs) as well as bevacizumab plus interferon-alpha are also commonly used in clinical practice based on subgroup analysis of larger phase 3 data, expanded access programs and retrospective data.[6,7,8,9] This is possibly due to potential availability and cost issues with temsirolimus as well as the need for weekly intravenous therapy, as opposed to the convenience associated with the oral TKIs.
This study was carried out to evaluate the performance of poor risk mRCC, as defined by Heng criteria, in the Indian context. To the best of our knowledge, this is the first such study regarding management strategies and survival of poor risk mRCC from India.
Materials and Methods
Database and patient population
This study was conducted as part of a prospective Clinical Trials Registry-India (CTRI) registered study, Kidney Cancer Registry, which is designed to collect data for patients with renal cell carcinoma (RCC) and was approved by the Institutional Review Board and Ethics Committee for all patients diagnosed with RCC (Institutional Ethics Committee-79; CTRI Number – CTRI/2012/11/003147). Patients diagnosed with metastatic RCC between February 2007 and August 2015 and who were potential candidates for treatment with systemic therapy were extracted from this database. These patients were then risk stratified by Heng criteria and those who fulfilled the criteria for “poor-risk” (3–6 risk factors) were included in this study.
Baseline demographics, comorbidities, disease status including metastatic burden and therapeutic options used, were studied. Grade 3 and Grade 4 adverse events (as per Common Terminology Criteria for Adverse Events version 4.0) were also enumerated.
Outcome variables
Response assessment for patients on active therapeutic intervention (excluding patients on best supportive care only) was done every 2–4 months by clinical evaluation and radiology, either computed tomography scans or positron emission tomography scans. Where feasible, response was assessed by response evaluation criteria in solid tumors (RECIST) criteria, version 1.1.[10] When the application of RECIST was not feasible, response was not quantified. Response rates (RRs) and clinical benefit rate (CBR) were reported as percentages. Event-free survival (EFS) was calculated as the time between the start of therapy and the date of progression, permanent cessation of drug, loss to follow-up or death from any cause (if the disease had not progressed). Overall survival (OS) was calculated as the time between the start of therapy and the date of death due to any cause or loss to follow-up.
Statistical analysis
Median EFS and median OS were estimated using Kaplan–Meier method. Eastern Cooperative Oncology Groups (ECOGs) performance status (PS) (0–1 vs. ≥2), age (>65 years vs. age ≤65 years), number of metastatic sites (<2>
Results
Baseline demographic and clinical characteristics
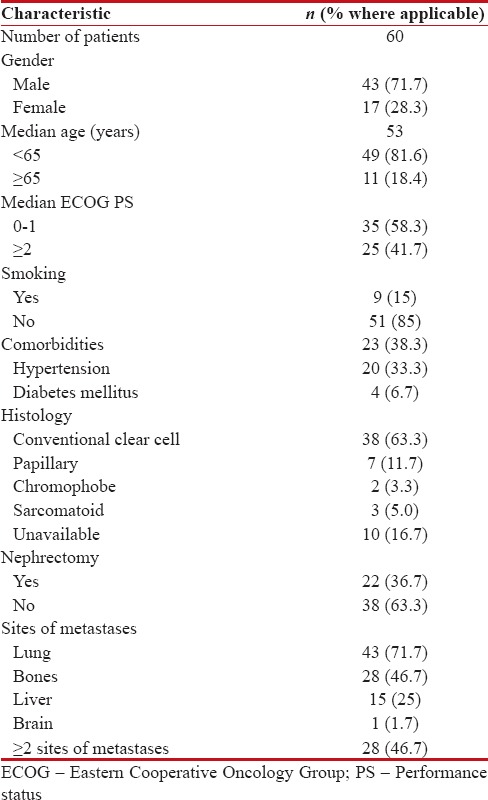
A total of sixty patients with poor risk mRCC were suitable for inclusion in analysis. Forty-three patients (71.7%) were male, median age was 53 years, with a median ECOG PS of 1. Histology was available in fifty patients (83.3%), with conventional clear cell histology seen in 38 (63.3%), papillary in 7 (11.7%), sarcomatoid in 3 (5.0%), and chromophobe in 2 (3.3%) patients, respectively. Twenty-two patients (36.7%) had undergone nephrectomy, and 28 patients (46.7%) had >2 sites of metastases [Table 1].
Table 1
Baseline characteristics
|
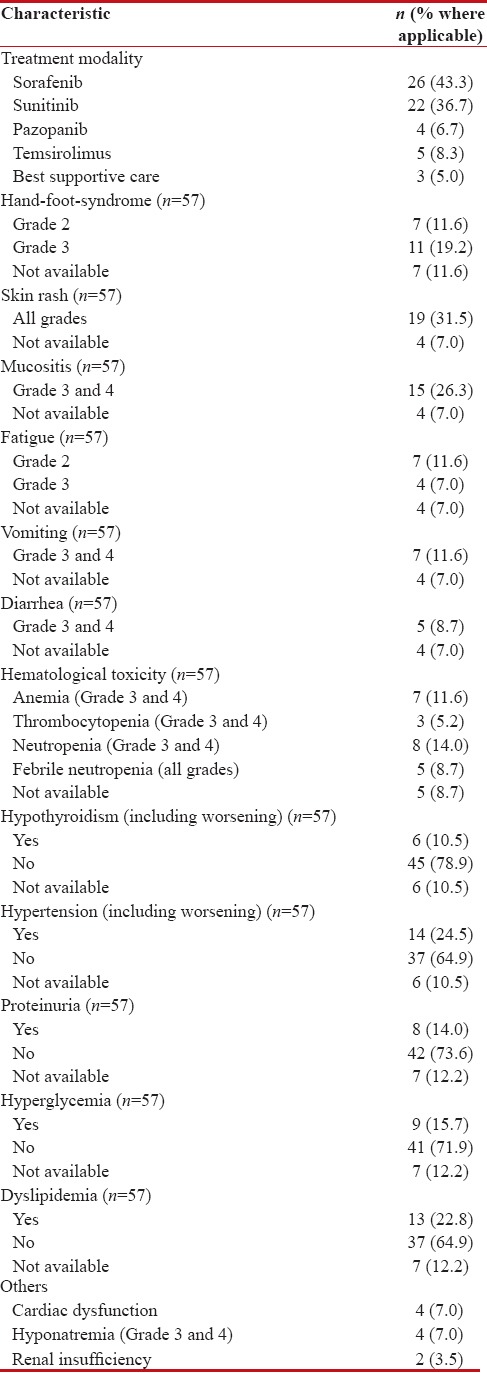
Treatment details and adverse event profile
Of the sixty patients, 26 (43.3%) received sorafenib, 22 (36.7%) sunitinib, 4 (6.7%) pazopanib, 5 (8.3%) temsirolimus, whereas three patients (5.0%) were offered best supportive care only [Table 2].
Table 2
Details of drug use and adverse events profile
|
Characteristics of the three patients offered best supportive upfront were:
- All three patients had an ECOG PS 3
- Two patients had nonclear cell histology while one patient had clear cell histology
- Two patients had ≥2 sites of metastases, whereas one patient has a single site of metastases.
Adverse events are reported for the entire cohort. The most common side effects were skin rash (all grades – 31.5%), hand-foot syndrome (HFS) (Grade 2 and Grade 3 – 30.8%), mucositis (26.3%), and fatigue (Grade 2 and Grade 3 – 18.6%). Metabolic adverse events seen commonly included hypertension (new onset as well worsening of preexisting hypertension – 24.5%), dyslipidemias (22.8%), and hyperglycemia (new onset as well worsening of preexisting hyperglycemia – 15.7%). Hematological adverse events were relatively less common, except anemia, seen in 11.6% of patients. Other rare side effects included renal insufficiency (3.5%), Grade 3/4 hyponatremia (7.0%), and symptomatic cardiac dysfunction (7.0%).
Response rates and survival data
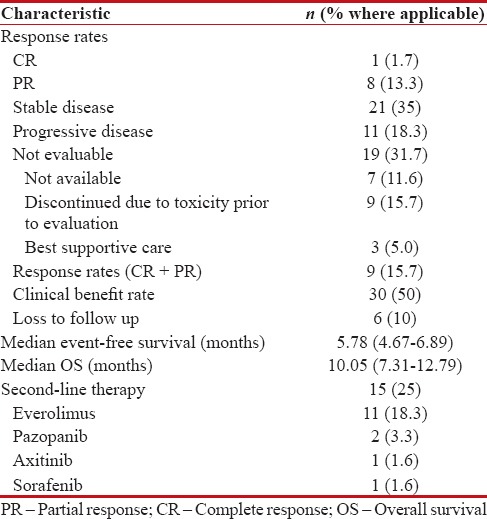
Of the entire of cohort of sixty patients, 41 patients (68.3%) were available for response assessment, whereas the remaining were unavailable for reasons specified in Table 3. One patient (1.7%) achieved a complete response, 13.3% of patients had achieved a partial response, whereas 35% of patients had stable disease for an overall RR of 15% and CBR of 50%. 18.3% of patients had progressive disease on the first evaluation post their respective intervention [Table 3].
Table 3
Response rates, survival, and second-line therapy
|
As of cut-off date for entry into analysis, with a median follow-up of 13.8 months (range 1–33 months), six patients (10%) were alive and on treatment, 48 patients (80%) had died due to disease, whereas six patients (10%) were lost to follow-up. Fifteen patients (25%) of patients were offered second-line therapy and details are as per Table 3.
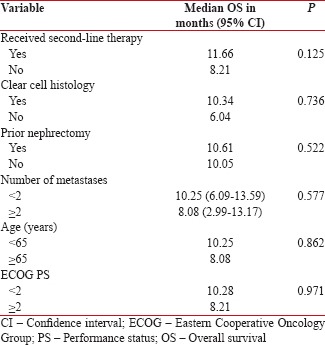
The overall estimated median EFS was 5.78 months (range 4.67–6.89 months) [Figure 1]. The overall estimated median OS in our study was 10.05 months (range 7.31–12.79) [Figure 2]. None of the factors evaluated as prognostic factors for OS including ECOG PS (0–1 vs. ≥2), age (<65 xss=removed>P = 0.125) [Supplement Table 1].
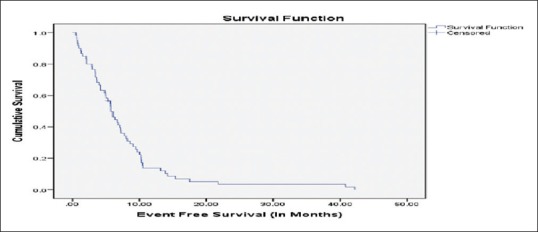
| Figure 1:Event-free survival in months

| Figure 2:Overall survival
Supplementary Table 1
Prognostic factors for overall survival
|
Discussion
The poor-risk category is a less well-studied cohort in the era of targeted agents being used in mRCC. They are either systematically excluded or under-represented in a majority of trials. For example, the seminal registration trials for sunitinib and pazopanib included only 6% and 3%, respectively, of patients stratified as a poor risk by MSKCC criteria.[11,12] Despite this under-representation in major trials, it does indicate that TKIs are feasible options in poor risk mRCC.
The patients in this study are representative of a real world population, as against a well-selected trial cohort. 41.7% of patients had an ECOG PS ≥2, 38.3% had at least one comorbidity, and 20% of them were of nonclear cell histology. Our study, by solely concentrating on this subset, attempts to provide an insight into how these patients are treated in an Indian tertiary cancer center. As background, a majority of our patients face financial constraints in affording temsirolimus, a 25 mg vial of which costs approximately INR 75,000/week (approximately US$1120). This is reflected in the management strategies at our center, where only 8.3% of our patients were treated with temsirolimus, while the remaining (excluding three patients planned for best supportive care only) received TKIs as first-line therapy. Despite the lack of randomized trial evidence for this approach, subgroup analysis from expanded access programs and retrospective data for sunitinib and sorafenib have shown a median progression-free survival (PFS) range of 3.9–5.4 months and an OS in the range of 6.4–9.3 months in poor risk patients.[3,7,11,13] To note, the seminal ARCC study showed a median PFS of 3.8 months and median OS of 10.9 months with single-agent temsirolimus. In comparison to these standards, the patients in our cohort had a median EFS of 5.7 months and median OS of 10.05 months. Since our study population had a small percentage of patients (8.3%) receiving temsirolimus, it would suggest that a majority of these outcomes can be attributed to the oral TKIs and as such, oral TKIs may be considered as an alternative to temsirolimus in Indian patients.
We noted high incidences of skin rash (all grades 31.5%), HFS (Grade 2 and Grade 3 – 30.8%) and surprisingly, metabolic adverse effects hypertension (24.5%), dyslipidemia (22.8%), and hyperglycemia (15.7%). The high incidence of metabolic side-effects is unexpected. There is growing evidence to suggest that changes in fasting glucose, triglyceride levels, and cholesterol levels could be used as pharmacodynamics biomarkers for mTOR inhibition.[14,15] However, a majority of our patients received oral TKIs, and a likelier reason for a high incidence of metabolic abnormalities might be unmasking of preexisting abnormalities during treatment. This also mandates watchfulness for and adequate treatment of these adverse events during treatment in Indian patients.
Patients exposed to the second line of therapy (25%), predominantly everolimus in our study, seemed to do better than those who were unable to receive the same and this approached but did not reach statistical significance (P = 0.125). This is in line with evidence which suggests that patients receiving second-line therapy may have prolonged survival to the tune of 12.5 months post first-line therapy.[16]
While our study and previously published data suggest that TKIs appear equivalent to temsirolimus for poor risk patients, the actual shift toward better management may come via molecules such as Nivolumab, a programmed death 1 immune checkpoint inhibitor antibody and cabozantinib, an oral TKI that targets VEGFR, MET, and AXL,[17,18] which have shown benefit across risk groups.
Our study is an addition to the literature on real world treatment of patients with poor-risk mRCC. However, being a retrospective analysis, certain caveats exits. We had a 10% lost to follow-up rate, as well as a lack of complete documentation of RRs. Toxicity data was based on retrieval of previously documented details, with an emphasis on only severe grades of toxicity rather than all grades. We also used the Heng criteria for selecting patients as poor-risk; a majority of published data have used the MSKCC criteria, thereby making cross – comparisons tenuous.
Conclusion
This study shows that oral TKIs may be considered as feasible alternatives to and along with Temsirolimus in patients with poor risk mRCC. The outcomes of low-risk mRCC patients in this study appear similar to published literature, although with a slightly different set of side-effects. Further studies with newer strategies are required to markedly improve outcomes in this under-evaluated subset of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Heng DY, Choueiri TK, Rini BI, Lee J, Yuasa T, Pal SK, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol 2014;25:149-54.
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40.
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794-9.
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81.
- Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii49-56.
- Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer 2015;113:12-9.
- Lee JL, Park I, Park K, Park S, Ahn Y, Ahn JH, et al. Efficacy and safety of vascular endothelial growth factor receptor tyrosine kinase inhibitors in patients with metastatic renal cell carcinoma and poor risk features. J Cancer Res Clin Oncol 2012;138:687-93.
- Escudier B, Bellmunt J, Négrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J Clin Oncol 2010;28:2144-50.
- Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J Clin Oncol 2013;31:3791-9.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
- Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma – NEJM. Available from: http://www.nejm.org/doi/full/10.1056/NEJMoa065044#t=article. [Last cited on 2016 Jun 27].
- Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer 2013;49:1287-96.
- Heng DY, Elson P, Golshayan AR, Warren MA, Kollmannsberger C, Chi KN, et al. A retrospective multicenter study of MSKCC poor-prognosis patients with metastatic renal cell carcinoma (mRCC) treated with sunitinib. 2008;26 15 Suppl:16057. [ASCO Meet Abstr].
- Nallari AS, Karrison T, Rosner GL, Levine MR, Sit L, Wu K, et al. Fasting glucose and triglycerides as biomarkers of mTOR inhibition, evidence of a categorical response. 2010;28 15 Suppl:3091. [ASCO Meet Abstr].
- Lee CK, Marschner IC, Simes RJ, Voysey M, Egleston B, Hudes G, et al. Increase in cholesterol predicts survival advantage in renal cell carcinoma patients treated with temsirolimus. Clin Cancer Res 2012;18:3188-96.
- Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: A population-based study. Lancet Oncol 2015;16:293-300.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-Cell carcinoma. N Engl J Med 2015;373:1803-13.
- Escudier BJ, Motzer RJ, Powles T, Tannir NM, Davis I, Donskov F. Subgroup analyses of METEOR, a randomized phase 3 trial of cabozantinib versus everolimus in patients (pts) with advanced renal cell carcinoma (RCC). J Clin Oncol 2016;34 2 Supplement:499. Available from: http://www.meetinglibrary.asco.org/content/158003-172. [Last cited on 2016 Aug 11].

| Figure 1:Event-free survival in months

| Figure 2:Overall survival
References
- Heng DY, Choueiri TK, Rini BI, Lee J, Yuasa T, Pal SK, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol 2014;25:149-54.
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40.
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794-9.
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81.
- Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii49-56.
- Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer 2015;113:12-9.
- Lee JL, Park I, Park K, Park S, Ahn Y, Ahn JH, et al. Efficacy and safety of vascular endothelial growth factor receptor tyrosine kinase inhibitors in patients with metastatic renal cell carcinoma and poor risk features. J Cancer Res Clin Oncol 2012;138:687-93.
- Escudier B, Bellmunt J, Négrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J Clin Oncol 2010;28:2144-50.
- Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J Clin Oncol 2013;31:3791-9.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
- Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma – NEJM. Available from: http://www.nejm.org/doi/full/10.1056/NEJMoa065044#t=article. [Last cited on 2016 Jun 27].
- Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer 2013;49:1287-96.
- Heng DY, Elson P, Golshayan AR, Warren MA, Kollmannsberger C, Chi KN, et al. A retrospective multicenter study of MSKCC poor-prognosis patients with metastatic renal cell carcinoma (mRCC) treated with sunitinib. 2008;26 15 Suppl:16057. [ASCO Meet Abstr].
- Nallari AS, Karrison T, Rosner GL, Levine MR, Sit L, Wu K, et al. Fasting glucose and triglycerides as biomarkers of mTOR inhibition, evidence of a categorical response. 2010;28 15 Suppl:3091. [ASCO Meet Abstr].
- Lee CK, Marschner IC, Simes RJ, Voysey M, Egleston B, Hudes G, et al. Increase in cholesterol predicts survival advantage in renal cell carcinoma patients treated with temsirolimus. Clin Cancer Res 2012;18:3188-96.
- Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: A population-based study. Lancet Oncol 2015;16:293-300.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-Cell carcinoma. N Engl J Med 2015;373:1803-13.
- Escudier BJ, Motzer RJ, Powles T, Tannir NM, Davis I, Donskov F. Subgroup analyses of METEOR, a randomized phase 3 trial of cabozantinib versus everolimus in patients (pts) with advanced renal cell carcinoma (RCC). J Clin Oncol 2016;34 2 Supplement:499. Available from: http://www.meetinglibrary.asco.org/content/158003-172. [Last cited on 2016 Aug 11].


 PDF
PDF  Views
Views  Share
Share

