PNET of kidney : Report of four cases
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(02): 130-133
DOI: DOI: 10.4103/0971-5851.99754
Abstract
Primitive neuroectodermal tumor (PNET) of kidney is a rare tumor of kidney with only a few published reports. We report here four cases of PNET of kidney in the age group between 30 and 50 years who had complaints of vague pain and lump in loin. Hematuria was present in one case. Imaging of all cases revealed renal mass. The pathologic findings were consistent with PNET in all cases-confirmed by immunohistochemistry with diffuse membrane positivity of tumor cells of CD99. We could not do fluorescent in situ hybridization to demonstrate EWS-FLI-1 gene fusion. Each case was in the advanced stage. However, after giving postoperative radiotherapy and chemotherapy patients are still alive. Reporting of these cases are important as we got them in a short span of 3 years. In view of its poor prognosis, aggressive nature and different therapeutic approach- renal PNET should be differentiated from other small blue round cell tumors like neuroblastoma, rhabdoid tumor of kidney, nephroblastoma, small cell carcinoma, synovial sarcoma (monophasic, poorly differentiated) and non-Hodgkin lymphoma (NHL) by immunohistochemistry, cytogenetic, and molecular genetics study to see the different gene rearrangements in NHL and 3p deletion in small cell carcinoma.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Primitive neuroectodermal tumor (PNET) of kidney is a rare tumor of kidney with only a few published reports. We report here four cases of PNET of kidney in the age group between 30 and 50 years who had complaints of vague pain and lump in loin. Hematuria was present in one case. Imaging of all cases revealed renal mass. The pathologic findings were consistent with PNET in all cases–confirmed by immunohistochemistry with diffuse membrane positivity of tumor cells of CD99. We could not do fluorescent in situ hybridization to demonstrate EWS-FLI-1 gene fusion. Each case was in the advanced stage. However, after giving postoperative radiotherapy and chemotherapy patients are still alive. Reporting of these cases are important as we got them in a short span of 3 years. In view of its poor prognosis, aggressive nature and different therapeutic approach– renal PNET should be differentiated from other small blue round cell tumors like neuroblastoma, rhabdoid tumor of kidney, nephroblastoma, small cell carcinoma, synovial sarcoma (monophasic, poorly differentiated) and non-Hodgkin lymphoma (NHL) by immunohistochemistry, cytogenetic, and molecular genetics study to see the different gene rearrangements in NHL and 3p deletion in small cell carcinoma.
INTRODUCTION
The peripheral primitive neuroectodermal tumor (PNET) was first described by Arthur Purdy Stout in 1918 and was included in the family of small round cell tumors. It commonly occurs in bones and is equivalent to Ewing's sarcoma microscopically, immunohistochemically, and genetically. Rarely, it can also occur as primary renal mass like other extraskeletal sites.[1] Most patients are young adults and clinical course is very aggressive. In kidney, most cases occur in the medullary/pelvic region.[2] Differential diagnosis includes monophasic Wilms’ tumor (blastema dominant), neuroblastoma, embryonal rhabdomyosarcoma, small-cell carcinoma, non-Hodgkin lymphoma and synovial sarcoma (monophasic, poorly differentiated). We here report four such cases that we got in our institution in a short span of 3 years that is much higher as compared to these cases in previous years in our institution.
CASE REPORTS
Case 1
In September 2006, a 34-year-old male presented with firm lump in right side of abdomen with mild pain for 6 months. On clinical examination, a round fixed mass (10×10 cm) was palpable in right hypochondrium. No history of hematuria was present. Computerized tomography (CT)-scan showed a large cystic mass in right kidney extending into retroperitoneum. Guided fine needle aspiration cytology (FNAC) was noncontributory due to large cystic spaces. Right radical nephrectomy was done with removal of associated lymphnodes. On gross examination, the specimen consisted of kidney with perirenal soft-tissue and adrenal of the right side. The mass was cystic, grayish-brown in color. Histologically, the tumor consisted of sheets of small round cells intervened by hemorrhagic cystic areas with surrounding thin rim of normal kidney tissue. Immunohistochemistry showed diffuse membrane positivity of tumor cells for CD99 and negative for cytokeratin, desmin, neuron-specific enolase (NSE) and CD45 (LCA). Patient also had ascites in which peritoneal fluid cytology showed the presence of malignant cells [Figure 1].
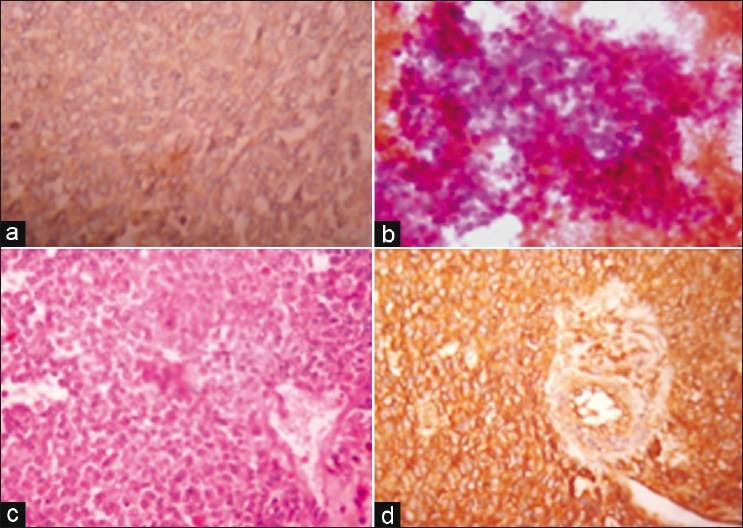
| Fig. 1 (a) Cytokeratin negative round cell tumor of kidney (IHC, ×400). (b) Peritoneal deposit of round cells (MGG, ×400). (c) Round cells’ rosette of PNET kidney (H and E, ×400). (d) Diffuse membrane CD99 positivity of round tumor cells (IHC, ×400)
Case 2
In October 2008, a 48-year-old male presented with pain in left loin for 2 years, low-grade fever for 3 months and hematuria for 1 month. Both ultrasonography (USG) and CT scan showed a large heterogeneous mass in upper pole of left kidney inseparable from tail of pancreas and spleen. Left radical nephrectomy was done with removal of adherent pancreas, descending colonic segment and seven lymphnodes. Grossly the kidney with tumor attained the size of 13.5 cm×9 cm×7 cm. Capsule was adherent with multiple grayish white nodules in perinephric fat. Ureter could not be identified [Figure 2]. Microscopically, the sections showed proliferation of small round cells in nests and rosettes diffusely infiltrating into renal capsule and perinephric fat, para-aortic lymphnodes, tail of pancreas and spleen. However, colonic segment was free. The tumor cells were positive for CD99, but negative for LCA, Cytokeratin, NSE. Therefore, PNET of kidney was confirmed.
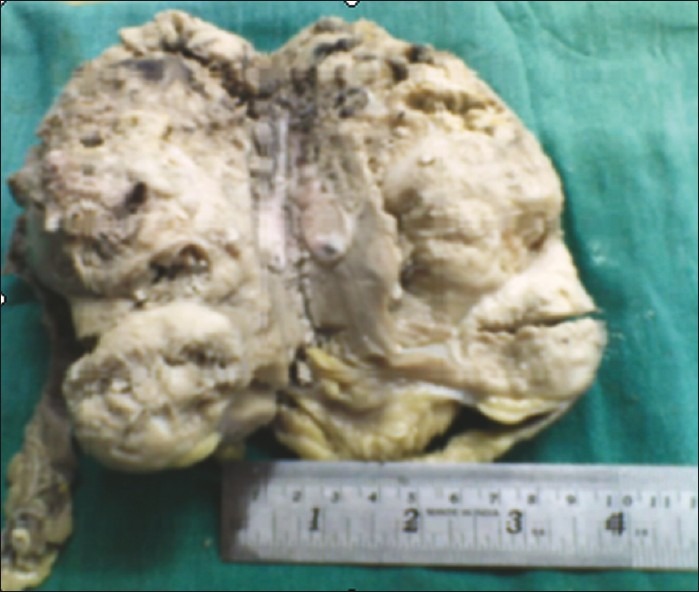
| Fig. 2 Gross features of PNET kidney showing grayish tumor in one pole
Case 3
In May 2009, a 50-year-old female presented with pain and heaviness in left loin for last 9 months but without hematuria. USG and CT scan report revealed left renal mass (16 cm×10 cm×8 cm) with thrombus in inferior vena cava (IVC) and retroperitoneal lymphadenopathy. Left-sided radical nephrectomy with removal of lymphnodes was done. Tumor thrombi in IVC and left renal vein were removed but no segment of affected veins was excised. Histologically, tumor was composed of small round cells in sheets and rosettes with metastasis in retroperitoneal lymphnodes. The tumor cells showed diffuse membrane positivity for CD99, but were negative for Cytokeratin, LCA, NSE, and chromogranin-A by immunohistochemistry. The diagnosis was confirmed as PNET.
Case 4
In January 2010, a 38-year-old female presented with vague pain in left loin with occasional hematuria for 5 months. On examination, a ballotable lump was felt in the left lumber region with mild tenderness. CT-scan revealed a left renal mass in medullary region (10×6 cm) with focal cystic changes. Perinephric tissue was free. Initially diagnosis was done by trucut biopsy and its routine hematoxylin eosin (H/E) stain and immunohistochemistry for CD99 and bcl- 2, considering synovial sarcoma as differential diagnosis. Left kidney with left hilar lymphnodes was removed. Grossly-tumor was confined within central portion of kidney, grayish-brown in color, with areas of hemorrhage and cystic changes. Only three nodes were enlarged. Microscopically, the tumor was composed of small round cells arranged in sheets and rosettes with high mitosis, areas of hemorrhage, and necrosis. Lymph nodes showed metastatic deposit by similar cells. Immunohistochemically, the tumor cells showed diffuse membrane positivity for CD99 and negativity for Cytokeratin, NSE, bcl-2 and LCA. The diagnosis was made as PNET [Figure 3].
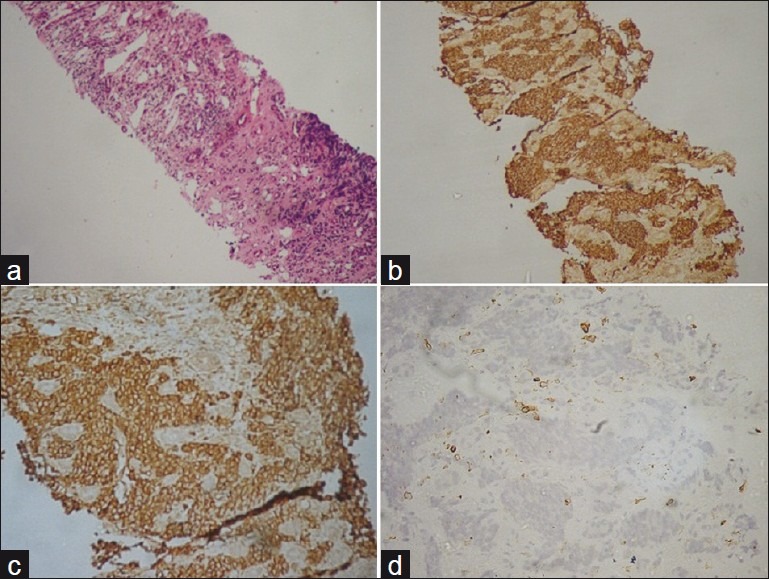
| Fig. 3 (a) Trucut biopsy of kidney tissue infiltrated by round cells of PNET (H and E, ×100). (b) Round cells of same trucut showing diffuse membrane positivity of CD99 (IHC, ×100). (c) Round cells of trucut showing diffuse membrane positivity of CD99 (IHC, ×200). (d) Round cells of same trucut tissue showing bcl-2 marker negativity (IHC, ×200)
DISCUSSION
PNET of soft tissue was traditionally regarded as equivalent to Ewing's Sarcoma of bone. The pathogenetic unity of these two types are confirmed by similar genetic aberrations. Bony counterpart is more undifferentiated than soft tissue, which shows neuroectodermal differentiation. PNET shows strong positivity for MIC-2 gene product like CD-99 over membrane of tumor cells and HBA-71 by which it can be distinguished from other small round cell tumors. If needed, its diagnosis can be confirmed by demonstration of the t(11:22) or the EWS-FLI-1 gene fusion with the help of fluorescent in situ hybridization (FISH) technique or RT-PCR.[3,4] A recent analysis of 146 cases of primary malignant neuroepithelial tumors of kidney from the National Wilms’ Tumor Study Group pathology center files led the authors to the conclusion that these malignancies represent a diverse group of high-grade tumor which is not always easy to place in a single category, even though they are evaluated immunohistochemically and by molecular genetic tools.[5]
The clinical features of PNET kidney may be nonspecific like vague pain and lump in the left loin and irregular fever, weight loss, and occasional hematuria. In our series, three cases had similar presentations. There is no sex predilection of this tumor, as was in our four cases male: Female ratio was 1:1. The age group of disease presentation is in the middle age. In our cases also, patients were in between 34 and 48 years of age. Diagnosis with TNM staging was done by routine USG screening followed by CT scan. Most cases can be diagnosed cytologically as small blue round cell tumors by guided FNAC or trucut biopsy. If there is large cystic spaces, cytologic evaluation may be difficult as we felt in one case.
Diagnosis and pathologic staging is confirmed by histopathology and immunohistochemistry in nephrectomy and trucut specimens. As the differential diagnosis of PNET of kidney include other small round cell tumors like neuroblastoma, adult nephroblastoma, malignant lymphoma, rhabdoid tumor, small cell carcinoma and monophasic, poorly differentiated, round cell variant of synovial sarcoma—immunohistochemical markers to be used to confirm diagnosis are cytokeratin (for nephroblastoma, small cell carcinoma, and synovial sarcoma), LCA (for Lymphoma), NSE/chromograninA (for neuroblastoma) and CD-99 for PNET. The histologic characteristic, Homer–Wright rosettes, commonly scarce or less defined in extraskeletal Ewing's sarcoma, is a typical feature of PNET and can help to consider the diagnosis. Although they can also be found in neuroblastoma. In our cases, we used the immunohistochemical markers mentioned and in one case bcl-2 marker additionally considering synovial sarcoma and all four cases showed diffuse positivity for CD-99 and negative for other markers. Though in some cases of PNET, the tumor cells may show NSE positivity as seen in neuroblastoma, the confirmation of PNET should be done by demonstrating CD99 positivity where the neuroblastoma cells are negative. CD99 positivity often are seen in synovial sarcoma which may be seen in kidney also. However, synovial sarcoma has characteristic morphology, bcl-2 marker and cytokeratin positivity. Moreover, cytogenetic studies (not done in our cases because of nonaffordability by the poor patients due to high cost of FISH and RT-PCR (reverse transcriptase polymerase chain reaction) to demonstrate different translocations related to PNET, synovial sarcoma, non-Hodgkin lymphoma, small cell carcinoma) are corroborative. As the tumor is highly aggressive, it is often diagnosed in advanced stage when it has already involved perinephric fat, hilar lymphnodes, renal veins, and inferior vena cava. In more advanced stages, the tumor involves liver, spleen, peritoneum, and lungs. The 5-year disease-free survival rate is around 45–55% in well-confined cases, whereas cases with advanced stage at presentation have a median relapse-free survival of only 2 years.[6]
Amongst our four cases all had involvement of hilar lymphnodes of same side, one case was confined within renal parenchyma and one had liver and peritoneal metastasis. In all our reported cases, the patients were given postoperative chemotherapy of vincristine, mesna, ifosfamide, and doxorubicin as initial regime in six cycles followed by radiotherapy; and after 1 year follow-up- vincristine, dactinomycin, etoposide, and ifosfamide as maintenance therapy, if CT scan or positron emission tomography (PET) scan reveals the persistence of tumor. However, with the mentioned regime, the patients are still alive without relapse. It is also important to differentiate renal PNET from renal embryonal rhabdomyosarcoma by using proper immunohistochemical markers besides previously mentioned tumors. Weeks et al. reported eight such cases of confirmed PNET having morphological similarity with rhabdomyosarcoma.[7] Although in both the conditions clinicopathologic features are same, but rhabdomyosarcoma occurs in younger with a more aggressive course. Rodriguez et al. postulated that these two renal neoplasms share a common undifferentiated stem cell precursors to explain their similarity.[8,9]
CONCLUSIONS
Exact diagnosis of PNET of kidney should be done using immunohistochemical markers and genetic markers to adopt appropriate management protocol with the hope of good clinical consequences. As the specific genetic defect can be identified, genetic therapy creating antisense oligonucleotides against the EWS-FLI-1 fusion gene will have good results to prolong the survival of patients. From our study, it can be suggested that in the setting of unavailability of molecular genetic analysis, clinicopathological features with immunohistochemical markers including suitable panel like CD99, cytokeratin, NSE, Desmin, LCA, bcl-2, can substantiate diagnosis of PNET of kidney.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 (a) Cytokeratin negative round cell tumor of kidney (IHC, ×400). (b) Peritoneal deposit of round cells (MGG, ×400). (c) Round cells’ rosette of PNET kidney (H and E, ×400). (d) Diffuse membrane CD99 positivity of round tumor cells (IHC, ×400)

| Fig. 2 Gross features of PNET kidney showing grayish tumor in one pole

| Fig. 3 (a) Trucut biopsy of kidney tissue infiltrated by round cells of PNET (H and E, ×100). (b) Round cells of same trucut showing diffuse membrane positivity of CD99 (IHC, ×100). (c) Round cells of trucut showing diffuse membrane positivity of CD99 (IHC, ×200). (d) Round cells of same trucut tissue showing bcl-2 marker negativity (IHC, ×200)


 PDF
PDF  Views
Views  Share
Share

