Pembrolizumab Weight-Based Dosing: Conviction and Lacunae in Adopting a Cost-Saving Approach—A Survey Report
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(03): 298-303
DOI: DOI: 10.1055/s-0042-1745814
Abstract
Introduction Use of immunotherapy drugs has increased leaps and bounds in the last decade with promising results in some of the cancers. The use is limited in low- and middle-income countries due to cost constraints. Weight-based dosing is one measure adopted by Canada and Israel to reduce cost burden and improve access to immunotherapeutic drugs.
Objective We conducted a survey among medical oncologists from India to understand challenges faced in accepting the weight-based dosing of pembrolizumab.
Materials and Methods Questionnaire covering various aspects related to use of immunotherapy drugs was made and it was circulated across various social media platforms. Medical oncologists practicing across India were invited to participate in this survey. The issues like access to drugs and awareness about weight-based dosing of pembrolizumab were covered in the survey. Also, the impact of international guidelines on accepting the weigh-based dosing was studied.
Results Ninety-nine medical oncologists across India participated in the survey. Only 60% medical oncologists are aware about weight-based dosing of pembrolizumab practiced in other countries. Further, 70% of medical oncologists could not prescribe immunotherapy due to cost factor in majority (90%) of their patients. More than 90% agreed that they will use weight-based dosing of pembrolizumab if the Drug Controller General of India, National Comprehensive Cancer Network, or European Society of Medical Oncologists guidelines endorses weight-based dosing.
Conclusion Weight-based dosing of pembrolizumab would be accepted if policy makers and Indian medical oncology societies come together and formulate guidelines. Such guidelines will improve accessibility to immunotherapy drugs and lead to huge cost savings.
Publication History
Article published online:
20 May 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Use of immunotherapy drugs has increased leaps and bounds in the last decade with promising results in some of the cancers. The use is limited in low- and middle-income countries due to cost constraints. Weight-based dosing is one measure adopted by Canada and Israel to reduce cost burden and improve access to immunotherapeutic drugs.
Objective We conducted a survey among medical oncologists from India to understand challenges faced in accepting the weight-based dosing of pembrolizumab.
Materials and Methods Questionnaire covering various aspects related to use of immunotherapy drugs was made and it was circulated across various social media platforms. Medical oncologists practicing across India were invited to participate in this survey. The issues like access to drugs and awareness about weight-based dosing of pembrolizumab were covered in the survey. Also, the impact of international guidelines on accepting the weigh-based dosing was studied.
Results Ninety-nine medical oncologists across India participated in the survey. Only 60% medical oncologists are aware about weight-based dosing of pembrolizumab practiced in other countries. Further, 70% of medical oncologists could not prescribe immunotherapy due to cost factor in majority (90%) of their patients. More than 90% agreed that they will use weight-based dosing of pembrolizumab if the Drug Controller General of India, National Comprehensive Cancer Network, or European Society of Medical Oncologists guidelines endorses weight-based dosing.
Conclusion Weight-based dosing of pembrolizumab would be accepted if policy makers and Indian medical oncology societies come together and formulate guidelines. Such guidelines will improve accessibility to immunotherapy drugs and lead to huge cost savings.
Introduction
Immunotherapy is a major development in the field of cancer care and therapeutics in the last decade. First immunotherapy drug ipilimumab got approval in 2011 for advanced melanoma after showing improved survival in phase 3 trial (NCT00324155).[1] Since then, these drugs are used in almost all cancers. It has improved outcome in few of the advanced cancers like lung, kidney, and liver. At any given time, hundreds of trials of immunotherapy drugs are ongoing across the world. These drugs are slowly being introduced into both adjuvant and neoadjuvant settings. This has led to accelerated approvals for these drugs in various advanced and early stage cancers. But this progress is limited to high-income countries. In an era of global cancer equality, patients from low- and middle-income countries (LMICs) are not able to access these drugs due to cost constraints. Health insurance coverage (both government and private) is limited in LMIC. In India, in 2014, t h e mean out-of-pocket expenditure for cancer treatment was Indian rupee (INR) 57,232. The expenditure is catastrophic for 79% of households (>10% of household consumption expenditure) and for 43% of them it is distress financing, meaning forced sale of house or land, borrowing money, or getting contributions from friends or families.[2] The study from Tata Memorial Hospital, Mumbai, revealed that only 1.6% of patients received immunotherapy.[3] Efforts are needed to reduce the cost of cancer therapy in LMIC. Various ways to reduce the cost for immunotherapy drugs need to be explored.
Use of weight-based dosing of pembrolizumab is one such way. Pembrolizumab was used by the manufacturing company in initial trials as 2 mg per kg every 3-weekly dose.[4] [5] [6] At that time, both 100 mg and 50 mg vials were available. But after few years, the company withdrew the 50 mg vial from the market. They also changed the dosing to fixed dose of 200 mg given 3-weekly. Now, this has led to the use of extra dose of drug especially for those patients who have average weight of 50–60 kg. It is wastage of money as same patient will need less doses in weight-based dosing as compared to a fixed-dose schedule. The pharmacokinetic studies done on pembrolizumab have shown that t h e d o s e s 2 mg per kg and 200 mg are equivalent in effect.[7] [8] [9] The dose of drugs is carefully decided after phase 1 studies. Both pharmacokinetic and pharmacodynamic properties of drug along with toxicity profile are taken into consideration while deciding final approved dose of the drug. The final maximum dose chosen for phase 3 clinical trials and subsequent drug approval is generally the one with better efficacy and with lesser toxicity. This is an old concept and needs revision in present time of targeted therapy and immunotherapy.[10]
According to a Canadian Agency for Drugs and Technologies in Health report, the Canadian authorities have approved the 2 mg/kg every 3-weekly dose of pembrolizumab across all tumors based on the basis of initial pembrolizumab clinical trials and modeling based studies.[8] With the establishment of the equal result of 2 mg/kg every 3 weeks and 200 mg every 3 weeks, there is potential of large cost savings on adopting the 2 mg/kg every 3 weeks as compared to the 200 mg flat dose.[9] The regulatory authorities have considered such weight-based strategies in Israel and Denmark. Many countries might follow the same in future. Indian regulatory guidelines from the Drug Controller General of India (DCGI) lack such recommendations.[11] To initiate similar efforts in India, we did this survey as to understand the difficulties faced by Indian medical oncologists (IMOs) in adopting weight-based immunotherapy.
Materials and Methods
We used Google Forms to conduct this survey and framed the questions according to the medical oncologist's perspective ([Table 1]). The survey was circulated through social media apps from August 1, 2021, to August 30, 2021. Only medical oncologists from across the country were included as survey participants. Survey participation was voluntary. Social media apps like WhatsApp and platforms such as Indian Society of Medical and Pediatric Oncology (ISMPO), the only official organization of medical oncologist in India, were used to circulate the survey questionnaires. All the responses to survey were collected in an Excel spreadsheet and analyzed. The questionnaire mainly focused on the primary objective of assessment of awareness of weight-based dosing of pembrolizumab among IMOs and understanding lacunae in adopting the same. Secondary objective was the assessment of knowledge of global efforts to reduce cost of immunotherapy drugs.
|
S. no |
Question |
Answers (options) |
Results: n (%) |
|---|---|---|---|
|
1 |
How many eligible patients of immunotherapy do not receive IO drug due to cost factor? |
1. >90% |
69 (69) |
|
2. 50–90% |
22 (22) |
||
|
3. <50> |
8 (8) |
||
|
2 |
Until 2015, pembrolizumab was studied at 2 mg/kg every 3 weeks in metastatic melanoma and mNSCLC. There is no rationale for 200 mg flat dose schedule for consequent trials. Do you agree? |
1. Yes |
85 (85) |
|
2. No |
5 (5) |
||
|
3. Do not know |
9 (9) |
||
|
3 |
What prohibits you from using pembrolizumab as mg/kg? |
1. Lack of recommendation by regulatory authorities |
15 (16)[a] |
|
2. Lack of recommendation from scientific organizations |
6 (6) |
||
|
3. Both options 1 and 2 |
43 (46) |
||
|
4. Lack of studies comparing 2 mg/kg versus flat 200 mg dose |
23 (25) |
||
|
5. Others |
7 (7) |
||
|
4 |
Are you aware of the fact that Canada, Israel, and Denmark have approved pembrolizumab as 2 mg/Kg every 3 weeks? |
1. Yes |
39 (39) |
|
2. No |
60 (60) |
||
|
5 |
If DCGI agrees to change its recommendation, would you change your practice to mg/Kg? |
1. Yes |
89 (89) |
|
2. No |
3 (3) |
||
|
3. Not sure |
7 (7) |
||
|
6 |
If NCCN adds its recommendation on mg/kg, would you change your practice? |
1. Yes |
93 (93) |
|
2. No |
1 (1) |
||
|
3. Not sure |
5 (5) |
||
|
7 |
If ESMO adds its recommendation on mg/kg, would you change your practice? |
1. Yes |
94 (94) |
|
2. No |
1 (1) |
||
|
3. Not sure |
4 (4) |
||
|
8 |
Are you a medical oncologist? |
1. Yes |
99 (100) |
|
2. No |
0 (0) |
||
|
9 |
How many of years of practice in medical oncology? |
1. 1–5 years |
44 (44) |
|
2. 6–10 years |
24 (24) |
||
|
3. >10 years |
31 (31) |
||
|
10 |
Where do you practice? |
1. Metro city |
74 (74) |
|
2. District |
14 (14) |
||
|
3. Towns |
11 (11) |
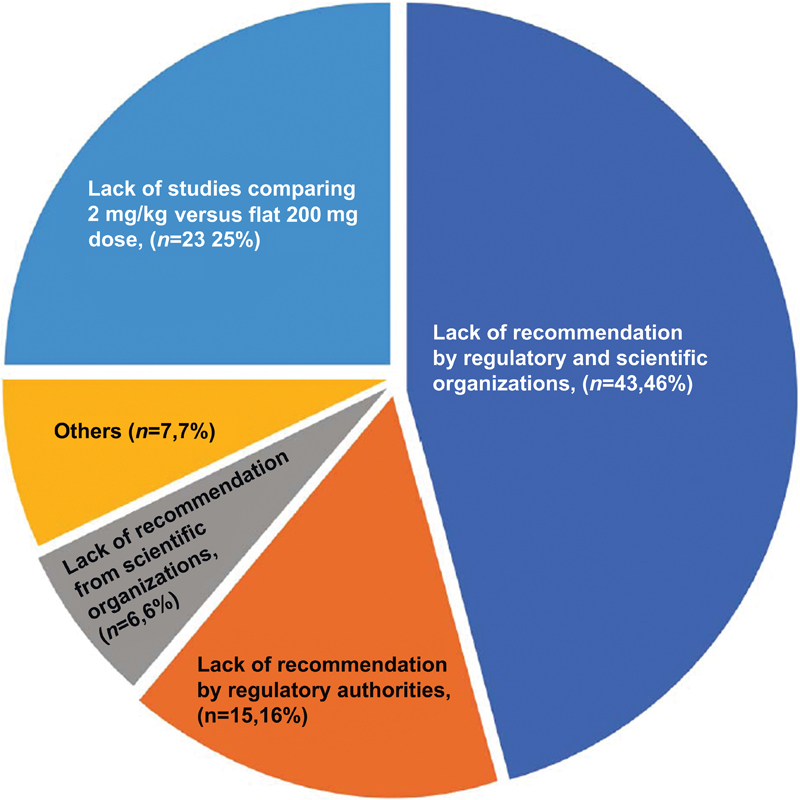
| Figure.2:Survey among Indian medical oncologist on factors prohibiting use of mg/kg dosing of pembrolizumab (n = 94).
Eighty five percent (n = 85) of IMOs agreed that there was no rationale for using only 200 mg every 3 weeks in subsequent trials of pembrolizumab. Sixty percent (n = 60) were not aware of the fact that countries like Canada, Israel, and Denmark have adopted the weight-based dose of pembrolizumab. Ninety percent IMOs would change their practice to weight-based dosing if DCGI recommends mg/kg dosing. Similarly, 93% and 94% of IMOs would change their practice if National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncologists (ESMO) add mg/kg recommendation of pembrolizumab in their guidelines ([Fig. 2]).
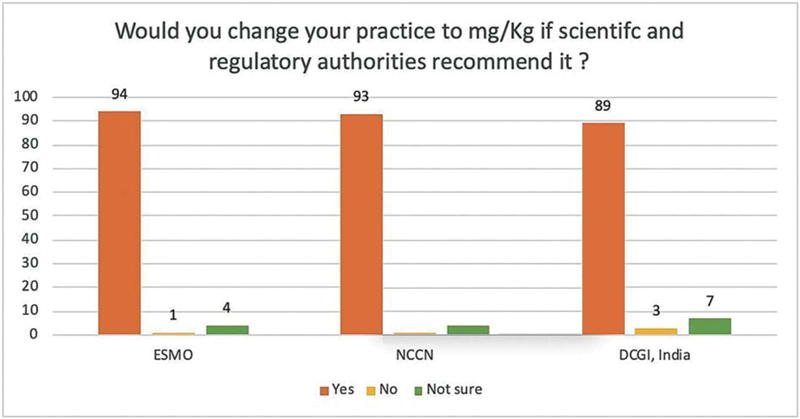
| Figure.2:Survey among Indian medical oncologists (n = 99) on changing the practice to using mg/kg dosing of pembrolizumab. ESMO, European Society of Medical Oncologists; NCCN, National Comprehensive Cancer Network; DCGI, Drugs Controller General of India.
Discussion
Most of the LMIC countries have no individual guidelines for cancer management.
Oncologists from LMICs depend heavily on NCCN or ESMO guidelines for treatment decisions in absence of any country-specific guidelines. As shown in our survey, almost all of the oncologists were reluctant to change the practice unless NCCN or ESMO guidelines recommend to do so. NCCN guidelines generally do not take into consideration financial stress caused by treatment, which is a vital deciding factor for treatment in LMIC patients. Recently, NCCN added the mg/kg dosing of pembrolizumab and nivolumab for malignant melanoma patients;[12] however, restricting such recommendations for other cancer indications is not well understood. This is a very important issue for change of practice at grassroots level. We also found that if Indian regulatory authorities, that is, DCGI, endorse such a recommendation, 90% of IMOs would change the practice. Hence, a coordinated effort is required between scientific organizations and drug approval authorities like Indian Council of Medical Research (ICMR) and the Central Drugs Standard Control Organization (CDSCO). The World Health Organization acknowledged the issue of lack of cancer treatment guidelines and lack of cost negotiations with the companies in LMICs.[13]
When pembrolizumab was launched, both the 100 mg and 50 mg vials were available. This was useful for administrating weight-based dosing. In 2017, the parent company withdrew 50 mg vial for reasons less understood. In fact, prescribing information for pembrolizumab provided by the company still has mention of 50 mg vial.[14] The average weight of an Indian lung cancer patient is lower as compared to western patients.[15] [16] For a mean weight of 55 kg, almost double dose of pembrolizumab is used if we use flat dose. Both pembrolizumab and nivolumab have shown comparable outcomes of weight based with a flat dose.[17] [18] [19] As per report published in 2017, pembrolizumab flat dosing leads to wastage of nearly 1 billion US dollar (USD) per year as 100 mg vial costs around INR 235,000 without access (USD 3,118).[9] The economic impact of weight-based dosing of pembrolizumab and nivolumab has been studied extensively and cost benefits are enormous including in the USA and European countries.[20] [21] [22] Negotiations must be done with the company for availability of 50 mg vial by the concerned regulatory authorities.
In our survey, IMOs that represented India's situation in medical oncology belonged to a diverse group of professionals from various social strata. They have faced the obstacle of limited access to the use of immunotherapy drugs. The results of such surveys as this can be useful for scientific organizations and also policy makers.
Limitations
Our survey is limited by the number of responders. India has almost 250–300 new medical oncologists coming into clinical practice every year and this survey was exclusive to them.
Conclusions
Weight-based dosing of pembrolizumab would be accepted if policy makers, regulative authorities, and IMOs come together and formulate the required guidelines. Such guidelines will improve accessibility of immunotherapy drugs and lead to huge cost savings. A coordinated effort is needed among scientific organizations like ISMPO, ICMR, and CDSCO (DCGI) to formulate India-centric guidelines on the use of pembrolizumab with weight-based (mg/kg) dose.
Conflict of Interest
None declared.
Acknowledgments
We thank all IMOs who participated in this survey. We are grateful to Dr. Daniel Goldstein for his support on sharing his experience on weight-based dosing of pembrolizumab. We also thank Dr. Navneet Singh for sharing his studies on body mass index in lung cancer.
References:
- Robert C, Thomas L, Bondarenko I. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364 (26) 2517-2526
- Kastor A, Mohanty SK. Disease-specific out-of-pocket and catastrophic health expenditure on hospitalization in India: Do Indian households face distress health financing?. PLoS One 2018; 13 (05) e0196106
- Noronha V, Abraham G, Patil V. et al. A real-world data of immune checkpoint inhibitors in solid tumors from India. Cancer Med 2021; 10 (05) 1525-1534
- Garon EB, Rizvi NA, Hui R. et al; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372 (21) 2018-2028
- Ribas A, Puzanov I, Dummer R. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16 (08) 908-918
- Herbst RS, Baas P, Kim D-W. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387 (10027): 1540-1550
- Centanni M, Moes DJAR, Trocóniz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 2019; 58 (07) 835-857
- Hafizi D, Eurich D. Canadian Agency for Drugs and Technologies in Health. CADTH technology review: optimal use 360 report: dosing and timing of immuno-oncology drugs. Published online November 2019. Accessed May 19, 2020 https://www.cadth.ca/sites/default/files/ou-tr/ho0008-dosing-timing-immuno-oncology-drugs.pdf
- Goldstein DA, Gordon N, Davidescu M. et al. A phamacoeconomic analysis of personalized dosing vs fixed dosing of pembrolizumab in firstline PD-L1-positive non-small cell lung cancer. J Natl Cancer Inst 2017;109(11):
- ;Shah M, Rahman A, Theoret MR, Pazdur R. The drug-dosing conundrum in oncology - when less is more. N Engl J Med 2021; 385 (16) 1445-1447
- Prabhash K, Vora A, Limaye S. et al. Treatment of advanced non-small-cell lung cancer: first line, maintenance, and second line–Indian consensus statement update (Under the aegis of Lung Cancer Consortium Asia, Indian Cooperative Oncology Network, Indian Society of Medical and Pediatric Oncology, Molecular Oncology Society, and Association of Physicians of India). Cancer Res Stat Treat 2021; 4 (02) 279-314
- National Comprehensive Cancer Network guidelines on cutaneous melanoma. Accessed on 2021, Oct 10. Available from https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- 23rd Expert Committee on Selection and Use of Essential Medicines [Internet]. Accessed March 7, 2022 from: https://www.who.int/news-room/events/detail/2021/06/21/default-calendar/23rd-expert-committee-on-selection-and-use-of-essential-medicines
- Keytruda prescribing information (package insert is based on worldwide physician circular S-CCDS-MK3475- IV-112017)..
- Singh N, Aggarwal AN, Gupta D, Behera D. Prevalence of low body mass index among newly diagnosed lung cancer patients in North India and its association with smoking status. Thorac Cancer 2011; 2 (01) 27-31
- Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Quantified smoking status and non-small cell lung cancer stage at presentation: analysis of a North Indian cohort and a systematic review of literature. J Thorac Dis 2012; 4 (05) 474-484
- Patnaik A, Kang SP, Rasco D. et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015; 21 (19) 4286-4293
- Chatterjee MS, Elassaiss-Schaap J, Lindauer A. et al. Population pharmacokinetic/pharmacodynamic modeling of tumor size dynamics in pembrolizumab-treated advanced melanoma. CPT Pharmacometrics Syst Pharmacol 2017; 6 (01) 29-39
- Chatterjee M, Turner DC, Felip E. et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016; 27 (07) 1291-1298
- Monirul S, Rigal M, Chouahnia K. et al. Budget impact analysis of fixed dose versus weight-based dosing regimen of nivolumab and pembrolizumab in the treatment of non-small cell lung cancer. Vaccines (Basel) 2020; 8 (04) 730
- Hall E, Zhang J, Kim EJ. et al. Economics of alternative dosing strategies for pembrolizumab and nivolumab at a single academic cancer center. Cancer Med 2020; 9 (06) 2106-2112
- Gamba T, Caglio A, Sacchi F. et al. Impact of different dosing strategies of nivolumab in patients with solid tumors: Italian single center analysis. Ann Oncol Res. 2021; 1 (01) 61
Address for correspondence
Publication History
Article published online:
20 May 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure.2:Survey among Indian medical oncologist on factors prohibiting use of mg/kg dosing of pembrolizumab (n = 94).

| Figure.2:Survey among Indian medical oncologists (n = 99) on changing the practice to using mg/kg dosing of pembrolizumab. ESMO, European Society of Medical Oncologists; NCCN, National Comprehensive Cancer Network; DCGI, Drugs Controller General of India.
References:
- Robert C, Thomas L, Bondarenko I. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364 (26) 2517-2526
- Kastor A, Mohanty SK. Disease-specific out-of-pocket and catastrophic health expenditure on hospitalization in India: Do Indian households face distress health financing?. PLoS One 2018; 13 (05) e0196106
- Noronha V, Abraham G, Patil V. et al. A real-world data of immune checkpoint inhibitors in solid tumors from India. Cancer Med 2021; 10 (05) 1525-1534
- Garon EB, Rizvi NA, Hui R. et al; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372 (21) 2018-2028
- Ribas A, Puzanov I, Dummer R. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16 (08) 908-918
- Herbst RS, Baas P, Kim D-W. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387 (10027): 1540-1550
- Centanni M, Moes DJAR, Trocóniz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 2019; 58 (07) 835-857
- Hafizi D, Eurich D. Canadian Agency for Drugs and Technologies in Health. CADTH technology review: optimal use 360 report: dosing and timing of immuno-oncology drugs. Published online November 2019. Accessed May 19, 2020 https://www.cadth.ca/sites/default/files/ou-tr/ho0008-dosing-timing-immuno-oncology-drugs.pdf
- Goldstein DA, Gordon N, Davidescu M. et al. A phamacoeconomic analysis of personalized dosing vs fixed dosing of pembrolizumab in firstline PD-L1-positive non-small cell lung cancer. J Natl Cancer Inst 2017;109(11):
- ;Shah M, Rahman A, Theoret MR, Pazdur R. The drug-dosing conundrum in oncology - when less is more. N Engl J Med 2021; 385 (16) 1445-1447
- Prabhash K, Vora A, Limaye S. et al. Treatment of advanced non-small-cell lung cancer: first line, maintenance, and second line–Indian consensus statement update (Under the aegis of Lung Cancer Consortium Asia, Indian Cooperative Oncology Network, Indian Society of Medical and Pediatric Oncology, Molecular Oncology Society, and Association of Physicians of India). Cancer Res Stat Treat 2021; 4 (02) 279-314
- National Comprehensive Cancer Network guidelines on cutaneous melanoma. Accessed on 2021, Oct 10. Available from https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- 23rd Expert Committee on Selection and Use of Essential Medicines [Internet]. Accessed March 7, 2022 from: https://www.who.int/news-room/events/detail/2021/06/21/default-calendar/23rd-expert-committee-on-selection-and-use-of-essential-medicines
- Keytruda prescribing information (package insert is based on worldwide physician circular S-CCDS-MK3475- IV-112017)..
- Singh N, Aggarwal AN, Gupta D, Behera D. Prevalence of low body mass index among newly diagnosed lung cancer patients in North India and its association with smoking status. Thorac Cancer 2011; 2 (01) 27-31
- Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Quantified smoking status and non-small cell lung cancer stage at presentation: analysis of a North Indian cohort and a systematic review of literature. J Thorac Dis 2012; 4 (05) 474-484
- Patnaik A, Kang SP, Rasco D. et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015; 21 (19) 4286-4293
- Chatterjee MS, Elassaiss-Schaap J, Lindauer A. et al. Population pharmacokinetic/pharmacodynamic modeling of tumor size dynamics in pembrolizumab-treated advanced melanoma. CPT Pharmacometrics Syst Pharmacol 2017; 6 (01) 29-39
- Chatterjee M, Turner DC, Felip E. et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016; 27 (07) 1291-1298
- Monirul S, Rigal M, Chouahnia K. et al. Budget impact analysis of fixed dose versus weight-based dosing regimen of nivolumab and pembrolizumab in the treatment of non-small cell lung cancer. Vaccines (Basel) 2020; 8 (04) 730
- Hall E, Zhang J, Kim EJ. et al. Economics of alternative dosing strategies for pembrolizumab and nivolumab at a single academic cancer center. Cancer Med 2020; 9 (06) 2106-2112
- Gamba T, Caglio A, Sacchi F. et al. Impact of different dosing strategies of nivolumab in patients with solid tumors: Italian single center analysis. Ann Oncol Res. 2021; 1 (01) 61


 PDF
PDF  Views
Views  Share
Share

