Pediatric Nonblastic Non-Hodgkin’s Lymphoma: A Perspective from India
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 13-17
DOI: DOI: 10.4103/ijmpo.ijmpo_42_16
Abstract
Background:?There is a paucity of data on pediatric nonblastic non-Hodgkin's lymphoma (NHL) from developing countries. We conducted this study to study outcome and identify risk factors that can predict survival in pediatric nonblastic NHL at our center.?Methods:?Patients <18 class="b" xss=removed>Results:?One hundred and two patients with median age of 12 years (range: 1?18) were included in the study. There were 69/102 (68%) male and 33/102 (32%) female patients. The most common histological diagnosis was Burkitt's lymphoma (BL) in 59/102 (58%) patients followed by anaplastic large cell lymphoma (ALCL) in 28/102 (28%) patients and diffuse large B-cell lymphoma (DLBCL) in 12/102 (12%) patients, T-cell lymphoma in 2/102 patients, and primary mediastinal B-cell lymphoma in 1/102 patients. The LMB-89 protocol was the most common protocol used for treatment in 74/102 (72%) patients. The 2-year event-free survival (EFS) for patients with BL, ALCL, and DLBCL was 72%, 55.8%, and 27.5%, respectively (P?= 0.037). On univariate analysis, factors that significantly predicted poor EFS included non-BL histological subtype, poor performance status, malnutrition, use of less intense chemotherapy, and not achieving complete response on interim radiological assessment. Conclusions: Outcomes in nonblastic NHL from our center are worse compared to data from the west. This is because a large proportion of patients present with advanced stage and in moribund condition. Patients with BL have better outcome compared to other subtypes.
Keywords
Chemotherapy - non-Hodgkin lymphoma - survival
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:?There is a paucity of data on pediatric nonblastic non-Hodgkin's lymphoma (NHL) from developing countries. We conducted this study to study outcome and identify risk factors that can predict survival in pediatric nonblastic NHL at our center.?Methods:?Patients <18 class="b" xss=removed>Results:?One hundred and two patients with median age of 12 years (range: 1?18) were included in the study. There were 69/102 (68%) male and 33/102 (32%) female patients. The most common histological diagnosis was Burkitt's lymphoma (BL) in 59/102 (58%) patients followed by anaplastic large cell lymphoma (ALCL) in 28/102 (28%) patients and diffuse large B-cell lymphoma (DLBCL) in 12/102 (12%) patients, T-cell lymphoma in 2/102 patients, and primary mediastinal B-cell lymphoma in 1/102 patients. The LMB-89 protocol was the most common protocol used for treatment in 74/102 (72%) patients. The 2-year event-free survival (EFS) for patients with BL, ALCL, and DLBCL was 72%, 55.8%, and 27.5%, respectively (P?= 0.037). On univariate analysis, factors that significantly predicted poor EFS included non-BL histological subtype, poor performance status, malnutrition, use of less intense chemotherapy, and not achieving complete response on interim radiological assessment. Conclusions: Outcomes in nonblastic NHL from our center are worse compared to data from the west. This is because a large proportion of patients present with advanced stage and in moribund condition. Patients with BL have better outcome compared to other subtypes.
Keywords
Chemotherapy - non-Hodgkin lymphoma - survivalIntroduction
More than 80% of children with pediatric non-Hodgkin's lymphoma (NHL) can be cured currently.[1] However, results of this success story seen in the developed world have not been replicated in developing countries.[2] There is a paucity of published literature on pediatric NHL from developing countries. We, therefore, conducted this study at our center to find the outcome of pediatric patients with nonblastic NHL and identify risk factors for disease relapse.
Methods
Data of all consecutive pediatric patients with newly diagnosed nonblastic NHL, <18 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_42_16#JR_3" xss=removed>3] Cytogenetic testing or molecular studies to identify specific translocations were not performed.
Patients with Burkitt's lymphoma (BL) were treated using the LMB-89 protocol, whereas patients with diffuse large B-cell lymphoma (DLBCL) were treated with either LMB-89 protocol or CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone) chemotherapy.[1] Patients with anaplastic large cell lymphoma (ALCL) were treated with either LMB-89 protocol or BFM-90 ALCL protocol or MCP-842 protocol.[1],[2],[4] Patients with primary mediastinal B-cell lymphoma (PMBCL) were treated with MACOP-B protocol.[5]
The Eastern Cooperative Oncology Group (ECOG) criteria were used for recording the performance status (PS) of the patients. However, the ECOG PS score is designed for adults and not for children. Patients were defined as moribund if they were severely malnourished and/or had disease-related complications such as hemodynamic instability, respiratory failure, bleeding diathesis, or altered sensorium making them unsuitable for intensive chemotherapy regimens. Moribund patients were treated with prednisolone and cyclophosphamide till their physical status improved, and they could tolerate more intensive chemotherapy. Undernutrition was defined as weight-for-age less than third centile on the World Health Organization growth charts.[6]
Event was defined as death due to any cause or relapse or progression of disease. Event-free survival (EFS) was calculated from date of initiation of treatment to date of relapse or documented progression or death. Patients were censored at the date of the event or date of last follow-up. EFS was estimated using Kaplan?Meier method and variables were compared using the log rank test. P < 0>
Results
During the study period, 102 patients with pediatric nonblastic NHL were treated at our hospital. The median age of the patients was 12 years (range: 1?18 years). There were 69 male and 33 female patients in the study. The duration of symptoms was <1 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_42_16#TB_1" xss=removed>Table 1].
|
Parameter |
n |
|---|---|
|
CNS ? Central nervous system; BM ? Bone marrow; DLBCL ? Diffuse large B-cell lymphoma; PMBCL ? Primary mediastinal B-cell lymphoma; PTCL ? Peripheral T-cell lymphoma; ALCL ? Anaplastic large-cell lymphoma; BFM ? Berlin-Frankfurt-Muenster |
|
|
Stage |
|
|
I |
11/102 |
|
II |
12/102 |
|
III |
62/102 |
|
IV |
17/102 |
|
Gender |
|
|
Male |
69/104 |
|
Female |
33/104 |
|
Age |
|
|
Median (range) |
12 (1-18) |
|
<5> |
23/102 |
|
5-10 years |
21/102 |
|
<10> |
58/102 |
|
Sites of disease |
|
|
Neck |
43 |
|
Thorax |
25 |
|
Abdomen |
73 |
|
CNS |
5 |
|
BM |
10 |
|
Bone |
5 |
|
Pathological subtype |
|
|
Burkitt?s lymphoma |
59/102 |
|
DLBCL |
12/102 |
|
PMBCL |
1/102 |
|
ALCL |
28/102 |
|
PTCL |
2/102 |
|
Chemotherapy protocol |
|
|
LMB-89 |
74 |
|
CHOP |
8 |
|
BFM-90 ALCL |
8 |
|
MACOP-B |
1 |
|
MCP-842 |
2 |
|
Cyclophosphamide and steroid |
6 |
|
Not treated |
3 |
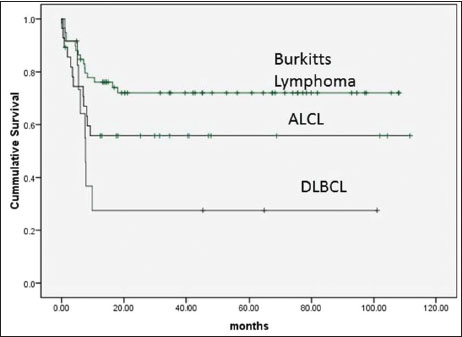
|?Figure.1Event-free survival according to pathological subtype
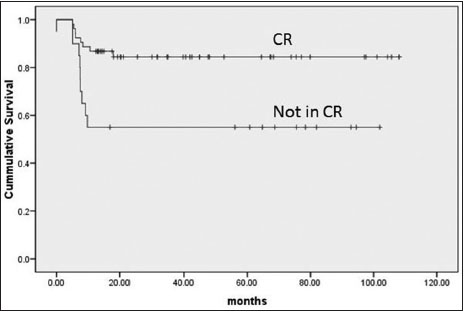
|?Figure.2Event-free survival according to interim assessment
|
Parameter (n) |
2-year EFS (SE) |
P* |
|---|---|---|
|
*Log rank test, +P < 0> |
||
|
Stage |
||
|
I (11) |
100 |
0.06 |
|
II (12) |
72.7 (13.4) |
|
|
III (62) |
55.4 (6.4) |
|
|
IV (17) |
58.8 (11.9) |
|
|
BL stage |
||
|
I (6) |
100 |
0.12 |
|
II (8) |
100 |
|
|
III (34) |
64 (8.4) |
|
|
IV (11) |
63.6 (14.5) |
|
|
ALCL stage |
||
|
I (4) |
100 |
0.4 |
|
II (3) |
33.3 (27.2) |
|
|
III (16) |
50 (12.5) |
|
|
IV (5) |
60 (21.9) |
|
|
Chemotherapy* |
||
|
Intensive (80) |
69.2 (5.1) |
0.002 |
|
Standard (19) |
33.7 (12.2) |
|
|
Gender |
||
|
Male (69) |
68.3 (5.8) |
0.08 |
|
Female (33) |
51.1 (8.8) |
|
|
Pathological subtype |
||
|
Burkitt?s (59) |
72.0 (6.0) |
0.01 |
|
DLBCL (12) |
27.5 (13.5) |
|
|
ALCL (28) |
55.8 (9.5) |
|
|
Interim assessment |
||
|
CR (53) |
84.4 (5.1) |
0.005 |
|
Not in CR (20) |
55.0 (11.1) |
|
|
Duration of symptoms |
||
|
Less than a month (50) |
61.1 (7.0) |
0.64 |
|
More than a month (52) |
64.0 (6.8) |
|
|
PS |
||
|
PS1 (53) |
72.8 (6.2) |
0.003 |
|
PS2 (30) |
62.2 (9) |
|
|
PS3 (15) |
30 (12.3) |
|
|
PS4 (2) |
50 (35.4) |
|
|
Undernourished |
||
|
Yes(36) |
52.3 (8.4) |
0.02 |
|
No (41) |
77.7 (6.6) |
|

|?Figure.1Event-free survival according to pathological subtype

|?Figure.2Event-free survival according to interim assessment
References
- atte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D.?et al.?The Soci?t? Fran?aise D'oncologie P?diatrique LMB89 protocol: Highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood 2001; 97: 3370-9
- dvani S, Pai S, Adde M, Vaidya S, Vats T, Naresh K.?et al.?Preliminary report of an intensified, short duration chemotherapy protocol for the treatment of pediatric non-Hodgkin's lymphoma in India. Ann Oncol 1997; 8: 893-7
- urphy SB, Fairclough DL, Hutchison RE, Berard CW.?Non-Hodgkin's lymphomas of childhood: An analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol 1989; 7: 186-93
- eidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G.?et al.?Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: A report of the Berlin-Frankfurt-M?nster Group Trial NHL-BFM 90. Blood 2001; 97: 3699-706
- limo P, Connors JM.?MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Intern Med 1985; 102: 596-602
- HO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization; 2006.
- hmad N, Zaidi A, Badar F, Maaz AU, Akram MS.?Clinical characteristics and outcome analysis of pediatric B-cell non-Hodgkin's lymphoma. Experience with FAB-LMB 96 and UKCCSG B-cell NHL guidelines in a developing country. Asia Pac J Clin Oncol 2010; 6: 49-56
- oleti ML, Al-Hadad SA, Al-Jadiry MF, Al-Darraji AF, Al-Saeed RM, De VellisA.?et al.?Treatment of children with B-cell non-Hodgkin lymphoma in a low-income country. Pediatr Blood Cancer 2011; 56: 560-7
- akhshi S, Radhakrishnan V, Sharma P, Kumar R, Vishnubhatla S.?et al.?Pediatric nonlymphoblastic non-Hodgkin lymphoma: Baseline, interim, and posttreatment PET/CT versus contrast-enhanced CT for evaluation ? A prospective study. Radiology 2012; 262: 956-68
- Lakshmaiah KC, Guruprasad B, Shah A, Kavitha S, Abraham LJ, Govindbabu K.?et al.?Anaplastic large cell lymphoma: A single institution experience from India. J Cancer Res Ther 2013; 9: 649-52
- Manipadam MT, Nair S, Viswabandya A, Mathew L, Srivastava A, Chandy M.?et al.?Non-Hodgkin lymphoma in childhood and adolescence: Frequency and distribution of immunomorphological types from a tertiary care center in South India. World J Pediatr 2011; 7: 318-25


 PDF
PDF  Views
Views  Share
Share

