Pediatric Acute Myeloid Leukemia in India: A Systematic Review
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(04): 342-348
DOI: DOI: 10.1055/s-0042-1754370
Abstract
Background Lower-middle-income countries face unique problems in the management of pediatric acute myeloid leukemia (AML) due to which the outcomes have not kept pace with developed nations. In India, data on childhood AML is sparsely available, thus making a true assessment of disease trends difficult. The current systematic review was undertaken to assess the outcomes of childhood AML from published literature from India over a period of 10 years (2011–2021).
Materials and Methods A systematic search of MEDLINE, Google Scholar, and SCOPUS was performed as per preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement from January 1, 2011 to December 31, 2021. In addition, International Society of Pediatric Oncology (SIOP) conference abstracts were also screened for relevant studies on AML from India. This study was registered in PROSPERO (ID42021273218).
Results A total of 1,210 patients from 19 studies were included. Standard 3 + 7 and MRC AML based regimens were commonly adopted regimens for induction. Remission rates varied between 56 and 95%. Overall treatment-related mortality across studies was 23.2% (95% confidence interval [CI]: 10.3–35.9%). The mean incidence of treatment abandonment was 19.3% ( 95% CI: 10.9–27.5%). Event-free survival and overall survival were in the range of 28 to 55% and 15 to 66%, respectively. Hematopoietic stem cell transplantation was performed only on a small subset of patients.
Conclusion Outcomes of pediatric AML in India continue to be suboptimal with high treatment abandonment and toxic deaths. Ensuring uniform access to therapy and supportive care along with a robust social support system would improve outcomes of childhood AML in India.
Keywords
acute myeloid leukemia - India - lower-middle-income countries - abandonmentOther Information
Protocol registration: PROSPERO (ID42021273218).
Competing Interests
The authors do not have any competing interests to declare.
All data that have been collected for the purpose of this systematic review have been from published literature and are available in public domains.
Authors' Contributions
Shyam Srinivasan was involved in conceptualization, designing, definition of intellectual content, literature search, clinical studies, experimental studies, data acquisition, data analysis, manuscript preparation, manuscript editing, and manuscript review.
Venkata Rama Mohan Gollamudi contributed to literature search and manuscript preparation.
Nidhi Dhariwal did literature search, data acquisition, and data analysis.
Supplementary MaterialPublication History
Article published online:
01 September 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background Lower-middle-income countries face unique problems in the management of pediatric acute myeloid leukemia (AML) due to which the outcomes have not kept pace with developed nations. In India, data on childhood AML is sparsely available, thus making a true assessment of disease trends difficult. The current systematic review was undertaken to assess the outcomes of childhood AML from published literature from India over a period of 10 years (2011–2021).
Materials and Methods A systematic search of MEDLINE, Google Scholar, and SCOPUS was performed as per preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement from January 1, 2011 to December 31, 2021. In addition, International Society of Pediatric Oncology (SIOP) conference abstracts were also screened for relevant studies on AML from India. This study was registered in PROSPERO (ID42021273218).
Results A total of 1,210 patients from 19 studies were included. Standard 3 + 7 and MRC AML based regimens were commonly adopted regimens for induction. Remission rates varied between 56 and 95%. Overall treatment-related mortality across studies was 23.2% (95% confidence interval [CI]: 10.3–35.9%). The mean incidence of treatment abandonment was 19.3% ( 95% CI: 10.9–27.5%). Event-free survival and overall survival were in the range of 28 to 55% and 15 to 66%, respectively. Hematopoietic stem cell transplantation was performed only on a small subset of patients.
Conclusion Outcomes of pediatric AML in India continue to be suboptimal with high treatment abandonment and toxic deaths. Ensuring uniform access to therapy and supportive care along with a robust social support system would improve outcomes of childhood AML in India.
Keywords
acute myeloid leukemia - India - lower-middle-income countries - abandonmentIntroduction
Acute leukemia accounts for approximately one-third of all childhood malignancies, of which 15 to 20%-cases-comprise of acute myeloid leukemia (AML).[1] The outcomes of childhood AML in high-income countries (HICs) have currently surpassed 70% with an increased focus on targeted therapies to further these outcomes and also simultaneously reduce toxicity.[2] [3] Lower-middle-income countries (LMICs) continue to have suboptimal outcomes due to various socioeconomic and disease-related factors.[3] There is limited data on childhood AML from India.[4] As a result, there is limited understanding of disease trends, which may ultimately compromise patient care. A previous systematic review from India, which included studies published between 1990 and 2010, highlighted several shortcomings of managing pediatric AML.[5] The current systematic review was undertaken to study the treatment strategies and outcomes of pediatric AML in India. The review included studies published between January 1, 2011 and December 31, 2021.
Materials and Methods
Protocol and Registration
This systematic review was registered on PROSPERO (ID42021273218).#
Eligibility Criteria
Inclusion Criteria
Studies reporting on pediatric AML in India.
Studies written in English.
Prospective, retrospective, and ambispective studies.
Exclusion Criteria
Studies on pediatric AML not from India.
Case reports, reviews, and books.
Settings
There were no restrictions on the type of setting in which the studies were conducted.#
Information Source
A systematic search of the MEDLINE, Google Scholar, and SCOPUS database for published studies on pediatric AML from India was conducted. In addition, SIOP conference abstracts were also screened. The reference lists of the included studies or relevant reviews were screened for other eligible studies.
Time
Search of database was from January 1, 2011 till January 31, 2021. SIOP conference abstracts were screened from year 2011 to 2020.
Literature Search
A comprehensive literature search was performed using text words “Acute myeloid leukemia,” “AML,” “child*,” “India.” Articles published in English alone were reviewed. Literature search was as per preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. The full search strategy is shown in [supplementary material].
Study Selection
Two review authors (S.S and V.R.M.G) independently screened the titles and abstracts yielded by the search against the inclusion and exclusion criteria. Full reports for all titles and abstracts were obtained if they appeared to meet the inclusion criteria and in case of any uncertainty. Review authors then screened the full text reports and decided whether the inclusion criteria were met. If necessary, additional information from study authors was sought to resolve questions about eligibility and disagreement was resolved through discussion. Reasons for excluding trials were also recorded. None of the review authors were blinded to the journal titles or to the study.
Data Collection Process
Data extraction from the included studies was performed using standardized data collection forms. Two reviewers (S.S and N.D) independently extracted the data to reduce the bias and errors in data extraction and the studies in question were jointly reviewed by the two investigators and the final determination was reached by consensus.
Data Items
The information that was extracted from each study included surname of the first author, year of study, median/mean age with range, number of patients, chemotherapy administered, induction mortality, complete remission (CR) rate, duration of follow-up, relapse, event-free survival (EFS), overall survival (OS), treatment-related mortality (TRM), treatment abandonment, prognostic factors, and use of hematopoietic stem cell transplant (HSCT).
Evaluation of Quality and Risk of Bias
Quality was assessed by two authors using the quality assessment tool for observational cohort and cross-sectional studies from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health.[6]
Synthesis Method
All studies included were screened for the required data items and results were tabulated using Microsoft Word software. Categorical variables were expressed as the number of cases and percentages (%). Mean along with 95%-confidence interval (CI) was calculated to report the incidence of TRM and abandonment rates. Statistical analyses were done using the R software version 4.0.2.
Results
Literature Search
A total of 1,057 studies and 15 SIOP conference abstracts were obtained after the initial search. Additionally, seven other studies were added after citation searching. After removing duplicates and screening the titles and abstracts of the publications, full text of 60 studies were assessed of which 19 were included for the systematic review. The PRISMA flowchart is shown in [Fig. 1].
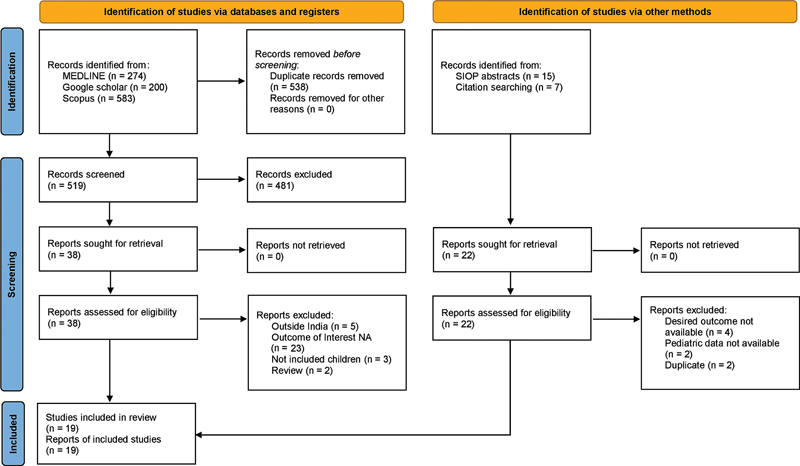
| Figure 1:Flow diagram of the systematic review according to preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.
Quality of Studies
The quality assessment tool for observational cohort and cross-sectional studies from the National heart, Lung, and Blood Institute of the National Institutes of Health was adapted to assess the quality of included studies ([Supplementary Table S1]).[7] Overall, the quality of the study was poor in 1 (5%) study, fair in 8 (42%) studies, and good in 10 (53%) studies of the 19 studies included in the systematic review.
Characteristics of the Studies
A total of 1,210 patients were included from the 19 studies.[8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] Three of the 19 studies also included patients with acute promyelocytic leukemia (APML).[19] [21] [22] Eight studies were published before 2015, while the remaining 11 studies were published in or after 2015. The various studies included patients between 1 and 19 years of age. There was slight predominance of males across majority of the studies. The salient features of the studies are summarized in [Table 1].
|
Study |
Type of study |
Time period |
Age (in years) |
M:F ratio |
Number of patients |
Chemotherapy |
Risk group included |
Median follow-up |
Abandonment (%) |
CR (%) |
Relapse/refractory disease (%) |
EFS/DFS |
Induction mortality (%) |
Overall TRM (%) |
OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Gupta et al 2011 [8] |
Retrospective |
2005–2009 |
Mean: 12.4 (1–18) |
1.9:1 |
35 |
In-I: 3 + 7 (D45mg/m2 + Arac 100mg/m2) In-II: HAM Consol: HIDAC |
All |
NA |
5.7% |
77.1% |
40% |
2 years DFS: 40% |
2.9% |
5.7% |
NA |
|
Yadav et al 2011 [9] |
Retrospective |
2005–2010 |
NA |
NA |
51 |
UKAML12 protocol |
All |
NA |
55% |
NA |
26% |
NA |
22% |
48% |
26% |
|
Mohammed et al 2013 [10] |
Retrospective |
2006–2013 |
NA |
NA |
34 |
NA |
All |
NA |
26% |
56% |
26% |
NA |
12% |
NA |
59% |
|
Kota et al 2013[11] |
Retrospective |
2007–2012 |
1–19 |
1:6:1 |
63 |
In: 3 + 7 Consol: NA |
NA |
11 months |
21% |
78% |
NA |
Median EFS: 11 months |
NA |
NA |
3 year OS: 15% |
|
Sharawat et al 2014 [12] |
Retrospective |
2008–2010 |
Median: 10(1–18) |
3:1 |
64 |
In: 3 + 7 (60mg/m2 *3 days) Consol: HIDAC |
All |
18.3 months |
NA |
83% |
NA |
EFS: 30.2 ± 5.8% DFS: 43.03 ± 7.3% |
NA |
NA |
37.1 ± 6.3% |
|
Jain et al 2014 [13] |
Retrospective |
2000–2013 |
NA |
NA |
88 |
In: 3 + 7 & 5 + 2 Consol: HIDAC + M |
NA |
NA |
34% |
NA |
NA |
NA |
NA |
18% |
NA |
|
Jayabose et al 2014 [14] |
Retrospective |
2010–2014 |
NA |
0.9:1 |
39 |
Modified MRC-10 protocol + M |
All |
29 months |
NA |
72% |
21.4% |
3 year EFS: 40% |
NA |
18% |
3 year OS: 47.5% |
|
Siddaiahgari et al 2014 [15] |
Prospective + Retrospective |
2009–2012 |
0.7:1 |
32 |
UK AML 15 protocol |
All |
NA |
NA |
94% |
16% |
NA |
NA |
6% |
72% |
|
|
Philip et al 2015[16] |
Retrospective |
2012–2014 |
NA |
NA |
23 |
AML-BFM 98 protocol + M |
NA |
7 months |
NA |
NA |
NA |
NA |
17% |
NA |
1 year OS: 70.4 ± 10.7% |
|
Radhakrishnan et al 2015 [17] |
Retrospective |
2008–2013 |
Median: 9 (1–17) |
2.25:1 |
72 |
In: DAE/DA Con: HIDAC |
All |
11.7 months |
NA |
72% |
NA |
EFS: 28% |
5.5% |
7% |
OS: 36% |
|
Ramamoorthy et al 2015 [18] |
Retrospective |
2004–2013 |
Mean: 7.3 ± 3.6 |
3.2:1 |
100 |
AML MRC 12 protocol |
All |
NA |
3% |
64% |
25% |
DFS: 34.7% |
25% |
48% |
27.2% |
|
Seth et al 2016 [19] |
Retrospective |
2011–2015 |
Median: 7.5 (1.5–13) |
NA |
71 (Included APML) |
MRC-10 protocol |
All |
NA |
25% |
95% (excluding APML) |
NA |
3 year EFS: 43% (excluding APML) |
5.4% |
27% |
3 year OS: 55% (excluding APML) |
|
Narula et al 2017 [20] |
Retrospective |
2011 |
NA |
NA |
65 |
In: 3 + 7 Consol: HIDAC + M |
All |
NA |
NA |
NA |
NA |
3 year DFS: 66% 3 year EFS: 49% |
<20> |
NA |
3 year OS:66% |
|
Naseer et al 2017 [21] |
Retrospective |
2012–2017 |
NA |
2:1 |
42 (included APML) |
In: 7 + 3 and 5 + 2 Con: HIDAC + M |
All |
NA |
9.4% |
56% |
25–28% |
NA |
18% |
NA |
19% |
|
Kapoor et al 2018[22] |
Retrospective |
2015–2018 |
NA |
NA |
24 (Included APML) |
In: 3 + 7 Consol: HIDAC |
All |
31 months |
4% |
NA |
29% |
NA |
NA |
NA |
67% |
|
Peyam et al 2018 [23] |
Retrospective |
2011–2017 |
Mean: 6.96(1–12) |
2.2:1 |
114 |
MRC 15 protocol |
All |
NA |
8.8% |
67.5% |
22.8% |
3 year EFS: 31.6% |
NA |
30.7% |
NA |
|
Sinha et al 2019[24] |
Retrospective |
2014–2015 |
<15> |
1.7:1 |
65 |
NA |
NA |
NA |
20% |
NA |
NA |
NA |
NA |
NA |
36.9% at 5 months after diagnosis |
|
Uppuluri et al 2020[25] |
Retrospective |
2002–2019 |
8 |
NA |
48 |
MRC 15 protocol |
All |
NA |
NA |
NA |
41% |
NA |
6.2% |
NA |
5 year OS:53% |
|
Srinivasan et al 2020 [26] |
Retrospective |
2014–2017 |
9 |
NA |
180 |
Upfront OMCT f/b 3 + 7 and HIDAC + M |
All |
25 months |
NA |
NA |
NA |
2 year EFS: 46–52 |
6.5% |
NA |
2 year OS: 47–53% |
References
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014; 64 (02) 83-103
- Chen J, Glasser CL. New and emerging targeted therapies for pediatric acute myeloid leukemia (AML). Children (Basel) 2020; 7 (02) 1-15
- Van Weelderen RE, Klein K, Natawidjaja MD, De Vries R, Kaspers GJ. Outcome of pediatric acute myeloid leukemia (AML) in low- and middle-income countries: a systematic review of the literature. Expert Rev Anticancer Ther 2021; 21 (07) 765-780 [Internet]
- Arora RS, Arora B. Acute leukemia in children: a review of the current Indian data. South Asian J Cancer 2016; 5 (03) 155-160
- Kulkarni KP, Marwaha RK. Childhood acute myeloid leukemia: an Indian perspective. Pediatr Hematol Oncol 2011; 28 (04) 257-268
- Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. [Internet]. [cited 2022 May 14]. Accessed July 12, 2022 from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies [cited 2022 January 5]. Accessed July 12, 2022 from: https://www.nhlbi.nih.gov/healthtopics/study-quality-assessment-tools.
- Gupta N, Seth T, Mishra P. et al. Treatment of acute myeloid leukemia in children: experience from a tertiary care hematology centre in India. Indian J Pediatr 2011; 78 (10) 1211-1215
- Yadav SP, Ramzan M, Lall M, Sachdeva A. Pediatric acute myeloid leukemia: final frontier for pediatric oncologists in developing world. Pediatr Hematol Oncol 2011; 28 (08) 647-648
- Mohammed R, Yadav SP, Meena L, Verma IC, Sachdeva A. PUB-0194, cytogenetic findings in acute myeloid leukemia: a developing country experience. Abstracts of the 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013. Hong Kong, China. September 25–28, 2013. Pediatr Blood Cancer 2013; 60 (Suppl. 03) 228
- Kota R, Linga VG, Gullipalli M. et al. PUB-0228, outcome of childhood acute myeloid leukaemia-Institutional experience from Indian subcontinent. Abstracts of the 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013. Hong Kong, China. September 25–28, 2013. Pediatr Blood Cancer 2013; 60 (Suppl. 03) 236
- Sharawat SK, Bakhshi R, Vishnubhatla S, Gupta R, Bakhshi S. FLT3-ITD mutation in relation to FLT3 expression in pediatric AML: a prospective study from India. Pediatr Hematol Oncol 2014; 31 (02) 131-137
- Jain K, Udgire S, Mudaliar S, Swami A, Shah N. EP-316, maintenance therapy in acute myeloid leukemia: experience from a developing country. 46(th) Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22(nd) -25(th) October, 2014 SIOP Abstracts. Pediatr Blood Cancer 2014; 61 (Suppl. 02) 324
- Jayabose S, Kasi VT, Vignesh SR, Priya R, Rathnam K. EP-317, Use of codified MRC-10 protocol for acute myeloblastic leukemia in Indian children. 46(th) Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22(nd) -25(th) October, 2014 SIOP Abstracts. Pediatr Blood Cancer 2014; 61 (Suppl. 02) 324
- Siddaiahgari S, Jillella B, Manikyam A. EP-327, Improving survival rates of acute myeloid leukemia in developing countries using AML_15 protocol. 46(th) Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22(nd) -25(th) October, 2014 SIOP Abstracts. Pediatr Blood Cancer 2014; 61 (Suppl. 02) 326
- Philip C, George B, Ganapule A. et al. Acute myeloid leukaemia: challenges and real world data from India. Br J Haematol 2015; 170 (01) 110-117
- Radhakrishnan V, Thampy C, Ganesan P. et al. Acute myeloid leukemia in children: experience from tertiary cancer centre in India. Indian J Hematol Blood Transfus 2016; 32 (03) 257-261
- Ramamoorthy J, Trehan A, Bansal D, Varma N, Jain R. PD-053, acute myeloid leukemia: treatment related mortality is abane in a developing country. 47th Congress of the International Society of Paediatric Oncology (SIOP) Cape Town, South Africa, October 8–11, 2015. Pediatr Blood Cancer 2015; 62 (Suppl. 04) 223
- Seth R, Pathak N, Singh A, Chopra A, Kumar R, Kalaivani M. Pediatric acute myeloid leukemia: improved survival rates in India. Indian J Pediatr 2017; 84 (02) 166-167 [Internet]
- Narula G, Prasad M, Jatia S. et al. Clinicoepidemiological profiles, clinical practices, and the impact of holistic care interventions on outcomes of pediatric hematolymphoid malignancies - a 7-year audit of the pediatric hematolymphoid disease management group at Tata Memorial Hospital. Indian J Cancer 2017; 54 (04) 609-615
- Naseer M, Ankit P, Purva K, Shraddha C. VP K. PO-012, acute myeloid leukemia: correlation of cytogenetics and outcome in a tertiary care pediatric centre. Abstracts From the 49th Congress of the International Society of Paediatric Oncology (SIOP) Washington, DC, USA October 12–15, 2017. Pediatr Blood Cancer. 2017; 64 (Suppl. 03) S442
- Kapoor R, Yadav SP. Genetics-based risk stratification of pediatric acute myeloid leukemia in India. Indian Pediatr 2018; 55 (11) 1006-1007
- Peyam S, Trehan A, Jain R, Bansal D, Varma N. PO-205, acute myeloid leukemia: incremental improvement in outcome in a developing country. Abstracts from the 50th Congress of the International Society of Paediatric Oncology (SIOP) Kyoto, Japan November 16–19, 2018. Pediatr Blood Cancer. 2018; (Suppl. 02) S186
- Sinha S, Brattström G, Palat G. et al. Treatment adherence and abandonment in acute myeloid leukemia in pediatric patients at a low-resource cancer center in India. Indian J Med Paediatr Oncol 2019; 40 (04) 501-506
- Uppuluri R, Swaminathan V, Ravichandran N. et al. Chemotherapy for childhood acute myeloid leukemia and associated infections over two decades in India: timeline and impact on outcome. Indian J Med Paediatr Oncol 2020; 41 (06) 869-873
- Srinivasan S, Dhamne C, Moulik N. , et al. 0037 / #975. A host-factor based approach impacts survival of children with acute myeloid leukemia (AML) at high-risk for induction mortality and/or early treatment abandonment. Abstracts from the 52th Congress of the International Society of Paediatric O. Pediatr Blood Cancer. 2020; 67 (Suppl. 04) S22
- Radhakrishnan V, Ganesan P, Rajendranath R, Ganesan TS, Sagar TG. Nutritional profile of pediatric cancer patients at Cancer Institute, Chennai. Indian J Cancer 2015; 52 (02) 207-209
- ;WHO estimates of acute leukemia.. Data [Internet][Cited on 2022 Jan 5]. Accessed July 12, 2022 from: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=356&key=asr&sex=0&cancer=39&type=0&statistic=5&
- Arora RS, Pizer B, Eden T. Understanding refusal and abandonment in the treatment of childhood cancer. Indian Pediatr 2010; 47 (12) 1005-1010
- Gupta S, Yeh S, Martiniuk A. et al. The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: a systematic review and meta-analysis. Eur J Cancer 2013; 49 (11) 2555-2564 [Internet]
- Atun R, Bhakta N, Denburg A. et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol 2020; 21 (04) e185-e224
- Jatia S, Narula G, Sankaran H. et al. Holistic support coupled with prospective tracking reduces abandonment in childhood cancers: a report from India. Pediatr Blood Cancer 2018; 2019: 1-8
- Howard SC, Zaidi A, Cao X. et al. The My Child Matters programme: effect of public-private partnerships on paediatric cancer care in low-income and middle-income countries. Lancet Oncol 2018; 19 (05) e252-e266 [Internet]
- Gibson BES, Webb DKH, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K. United Kingdom Childhood Leukaemia Working Group and the Dutch Childhood Oncology Group. Results of a randomized trial in children with acute myeloid leukaemia: medical research council AML12 trial. Br J Haematol 2011; 155 (03) 366-376
- Creutzig U, Zimmermann M, Reinhardt D, Michael D, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis. J Clin Oncol 2004; 2004 (22) 4384-4393
- Rubnitz JE, Kaspers GJL. How I treat pediatric acute myeloid leukemia. Blood 2021; 138 (12) 1009-1018
- Klein K, Van Litsenburg RRL, de Haas V. et al. Causes of early death and treatment-related death in newly diagnosed pediatric acute myeloid leukemia: recent experiences of the Dutch Childhood Oncology Group. Pediatr Blood Cancer 2019; 2020: 1-10
- Bansal D, Davidson A, Supriyadi E, Njuguna F, Ribeiro RC, Kaspers GJL. SIOP PODC adapted risk stratification and treatment guidelines: recommendations for acute myeloid leukemia in resource-limited settings. Pediatr Blood Cancer 2019; (October): e28087
- Sonowal R, Gupta V. Nutritional status in children with acute lymphoblastic leukemia, and its correlation with severe infection. Indian J Cancer 2021; 58 (02) 190-194
- Tandon S, Moulik NR, Kumar A, Mahdi AA, Kumar A. Effect of pre-treatment nutritional status, folate and vitamin B12 levels on induction chemotherapy in children with acute lymphoblastic leukemia. Indian Pediatr 2015; 52 (05) 385-389
- Singh R, Bakhshi S. (InPOG) e collaborative research in India comes of age. Pediatr Hematol Oncol J 2016; 1 (01) 13-17
Address for correspondence
Publication History
Article published online:
01 September 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Flow diagram of the systematic review according to preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.
References
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014; 64 (02) 83-103
- Chen J, Glasser CL. New and emerging targeted therapies for pediatric acute myeloid leukemia (AML). Children (Basel) 2020; 7 (02) 1-15
- Van Weelderen RE, Klein K, Natawidjaja MD, De Vries R, Kaspers GJ. Outcome of pediatric acute myeloid leukemia (AML) in low- and middle-income countries: a systematic review of the literature. Expert Rev Anticancer Ther 2021; 21 (07) 765-780 [Internet]
- Arora RS, Arora B. Acute leukemia in children: a review of the current Indian data. South Asian J Cancer 2016; 5 (03) 155-160
- Kulkarni KP, Marwaha RK. Childhood acute myeloid leukemia: an Indian perspective. Pediatr Hematol Oncol 2011; 28 (04) 257-268
- Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. [Internet]. [cited 2022 May 14]. Accessed July 12, 2022 from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies [cited 2022 January 5]. Accessed July 12, 2022 from: https://www.nhlbi.nih.gov/healthtopics/study-quality-assessment-tools.
- Gupta N, Seth T, Mishra P. et al. Treatment of acute myeloid leukemia in children: experience from a tertiary care hematology centre in India. Indian J Pediatr 2011; 78 (10) 1211-1215
- Yadav SP, Ramzan M, Lall M, Sachdeva A. Pediatric acute myeloid leukemia: final frontier for pediatric oncologists in developing world. Pediatr Hematol Oncol 2011; 28 (08) 647-648
- Mohammed R, Yadav SP, Meena L, Verma IC, Sachdeva A. PUB-0194, cytogenetic findings in acute myeloid leukemia: a developing country experience. Abstracts of the 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013. Hong Kong, China. September 25–28, 2013. Pediatr Blood Cancer 2013; 60 (Suppl. 03) 228
- Kota R, Linga VG, Gullipalli M. et al. PUB-0228, outcome of childhood acute myeloid leukaemia-Institutional experience from Indian subcontinent. Abstracts of the 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013. Hong Kong, China. September 25–28, 2013. Pediatr Blood Cancer 2013; 60 (Suppl. 03) 236
- Sharawat SK, Bakhshi R, Vishnubhatla S, Gupta R, Bakhshi S. FLT3-ITD mutation in relation to FLT3 expression in pediatric AML: a prospective study from India. Pediatr Hematol Oncol 2014; 31 (02) 131-137
- Jain K, Udgire S, Mudaliar S, Swami A, Shah N. EP-316, maintenance therapy in acute myeloid leukemia: experience from a developing country. 46(th) Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22(nd) -25(th) October, 2014 SIOP Abstracts. Pediatr Blood Cancer 2014; 61 (Suppl. 02) 324
- Jayabose S, Kasi VT, Vignesh SR, Priya R, Rathnam K. EP-317, Use of codified MRC-10 protocol for acute myeloblastic leukemia in Indian children. 46(th) Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22(nd) -25(th) October, 2014 SIOP Abstracts. Pediatr Blood Cancer 2014; 61 (Suppl. 02) 324
- Siddaiahgari S, Jillella B, Manikyam A. EP-327, Improving survival rates of acute myeloid leukemia in developing countries using AML_15 protocol. 46(th) Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22(nd) -25(th) October, 2014 SIOP Abstracts. Pediatr Blood Cancer 2014; 61 (Suppl. 02) 326
- Philip C, George B, Ganapule A. et al. Acute myeloid leukaemia: challenges and real world data from India. Br J Haematol 2015; 170 (01) 110-117
- Radhakrishnan V, Thampy C, Ganesan P. et al. Acute myeloid leukemia in children: experience from tertiary cancer centre in India. Indian J Hematol Blood Transfus 2016; 32 (03) 257-261
- Ramamoorthy J, Trehan A, Bansal D, Varma N, Jain R. PD-053, acute myeloid leukemia: treatment related mortality is abane in a developing country. 47th Congress of the International Society of Paediatric Oncology (SIOP) Cape Town, South Africa, October 8–11, 2015. Pediatr Blood Cancer 2015; 62 (Suppl. 04) 223
- Seth R, Pathak N, Singh A, Chopra A, Kumar R, Kalaivani M. Pediatric acute myeloid leukemia: improved survival rates in India. Indian J Pediatr 2017; 84 (02) 166-167 [Internet]
- Narula G, Prasad M, Jatia S. et al. Clinicoepidemiological profiles, clinical practices, and the impact of holistic care interventions on outcomes of pediatric hematolymphoid malignancies - a 7-year audit of the pediatric hematolymphoid disease management group at Tata Memorial Hospital. Indian J Cancer 2017; 54 (04) 609-615
- Naseer M, Ankit P, Purva K, Shraddha C. VP K. PO-012, acute myeloid leukemia: correlation of cytogenetics and outcome in a tertiary care pediatric centre. Abstracts From the 49th Congress of the International Society of Paediatric Oncology (SIOP) Washington, DC, USA October 12–15, 2017. Pediatr Blood Cancer. 2017; 64 (Suppl. 03) S442
- Kapoor R, Yadav SP. Genetics-based risk stratification of pediatric acute myeloid leukemia in India. Indian Pediatr 2018; 55 (11) 1006-1007
- Peyam S, Trehan A, Jain R, Bansal D, Varma N. PO-205, acute myeloid leukemia: incremental improvement in outcome in a developing country. Abstracts from the 50th Congress of the International Society of Paediatric Oncology (SIOP) Kyoto, Japan November 16–19, 2018. Pediatr Blood Cancer. 2018; (Suppl. 02) S186
- Sinha S, Brattström G, Palat G. et al. Treatment adherence and abandonment in acute myeloid leukemia in pediatric patients at a low-resource cancer center in India. Indian J Med Paediatr Oncol 2019; 40 (04) 501-506
- Uppuluri R, Swaminathan V, Ravichandran N. et al. Chemotherapy for childhood acute myeloid leukemia and associated infections over two decades in India: timeline and impact on outcome. Indian J Med Paediatr Oncol 2020; 41 (06) 869-873
- Srinivasan S, Dhamne C, Moulik N. , et al. 0037 / #975. A host-factor based approach impacts survival of children with acute myeloid leukemia (AML) at high-risk for induction mortality and/or early treatment abandonment. Abstracts from the 52th Congress of the International Society of Paediatric O. Pediatr Blood Cancer. 2020; 67 (Suppl. 04) S22
- Radhakrishnan V, Ganesan P, Rajendranath R, Ganesan TS, Sagar TG. Nutritional profile of pediatric cancer patients at Cancer Institute, Chennai. Indian J Cancer 2015; 52 (02) 207-209
- ;WHO estimates of acute leukemia.. Data [Internet][Cited on 2022 Jan 5]. Accessed July 12, 2022 from: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=356&key=asr&sex=0&cancer=39&type=0&statistic=5&
- Arora RS, Pizer B, Eden T. Understanding refusal and abandonment in the treatment of childhood cancer. Indian Pediatr 2010; 47 (12) 1005-1010
- Gupta S, Yeh S, Martiniuk A. et al. The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: a systematic review and meta-analysis. Eur J Cancer 2013; 49 (11) 2555-2564 [Internet]
- Atun R, Bhakta N, Denburg A. et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol 2020; 21 (04) e185-e224
- Jatia S, Narula G, Sankaran H. et al. Holistic support coupled with prospective tracking reduces abandonment in childhood cancers: a report from India. Pediatr Blood Cancer 2018; 2019: 1-8
- Howard SC, Zaidi A, Cao X. et al. The My Child Matters programme: effect of public-private partnerships on paediatric cancer care in low-income and middle-income countries. Lancet Oncol 2018; 19 (05) e252-e266 [Internet]
- Gibson BES, Webb DKH, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K. United Kingdom Childhood Leukaemia Working Group and the Dutch Childhood Oncology Group. Results of a randomized trial in children with acute myeloid leukaemia: medical research council AML12 trial. Br J Haematol 2011; 155 (03) 366-376
- Creutzig U, Zimmermann M, Reinhardt D, Michael D, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis. J Clin Oncol 2004; 2004 (22) 4384-4393
- Rubnitz JE, Kaspers GJL. How I treat pediatric acute myeloid leukemia. Blood 2021; 138 (12) 1009-1018
- Klein K, Van Litsenburg RRL, de Haas V. et al. Causes of early death and treatment-related death in newly diagnosed pediatric acute myeloid leukemia: recent experiences of the Dutch Childhood Oncology Group. Pediatr Blood Cancer 2019; 2020: 1-10
- Bansal D, Davidson A, Supriyadi E, Njuguna F, Ribeiro RC, Kaspers GJL. SIOP PODC adapted risk stratification and treatment guidelines: recommendations for acute myeloid leukemia in resource-limited settings. Pediatr Blood Cancer 2019; (October): e28087
- Sonowal R, Gupta V. Nutritional status in children with acute lymphoblastic leukemia, and its correlation with severe infection. Indian J Cancer 2021; 58 (02) 190-194
- Tandon S, Moulik NR, Kumar A, Mahdi AA, Kumar A. Effect of pre-treatment nutritional status, folate and vitamin B12 levels on induction chemotherapy in children with acute lymphoblastic leukemia. Indian Pediatr 2015; 52 (05) 385-389
- Singh R, Bakhshi S. (InPOG) e collaborative research in India comes of age. Pediatr Hematol Oncol J 2016; 1 (01) 13-17


 PDF
PDF  Views
Views  Share
Share

