PD-L1 Testing and Assessment: Practical Considerations for Oncologist and Pathologist
CC BY 4.0 · Indian J Med Paediatr Oncol 2024; 45(02): 157-162
DOI: DOI: 10.1055/s-0042-1748797
Introduction
Recently, immunotherapy with anti-PD-1(programmed cell death protein 1) or anti-PD-L1 (programmed cell death ligand 1) antibodies has shown both favorable and durable responses in a subset of patients with metastatic and advanced cancers. Although no robust predictive biomarker for immune checkpoint inhibitors (ICI) has been established till date, PD-L1 immunohistochemistry (IHC) testing has emerged with a passable utility. However, PD-L1 is still far from being a perfect biomarker. Nevertheless, PD-L1 testing by IHC to evaluate the immunoexpression of PD-L1 protein in tumor cells and/or immune cells is a useful predictive biomarker for predicting response to ICI.[1] [2] [3] [4]
Source of Support
None declared.
Publication History
Article published online:
28 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Introduction
Recently, immunotherapy with anti-PD-1(programmed cell death protein 1) or anti-PD-L1 (programmed cell death ligand 1) antibodies has shown both favorable and durable responses in a subset of patients with metastatic and advanced cancers. Although no robust predictive biomarker for immune checkpoint inhibitors (ICI) has been established till date, PD-L1 immunohistochemistry (IHC) testing has emerged with a passable utility. However, PD-L1 is still far from being a perfect biomarker. Nevertheless, PD-L1 testing by IHC to evaluate the immunoexpression of PD-L1 protein in tumor cells and/or immune cells is a useful predictive biomarker for predicting response to ICI.[1] [2] [3] [4]
Types of PD-L1 IHC Assays and Scoring
In oncology practice, the three most commonly used PD-L1 IHC assays, their respective PD-L1 antibodies, and associated IHC platforms are 22C3 (Dako), SP142 (Ventana), and SP263 (Ventana). A particular PD-L1 antibody clone and its associated platform have been approved by U.S. Food and Drug Administration (FDA) for respective ICI (PD-1 and PD- L1 inhibitor) intended for a particular malignancy type. Moreover, the approval also takes into account the type of cells expressing PD-L1, based on which the following three types of scoring ([Figs. 1] [2] [3] [4] [5] [6] [7] [8]) have been developed:

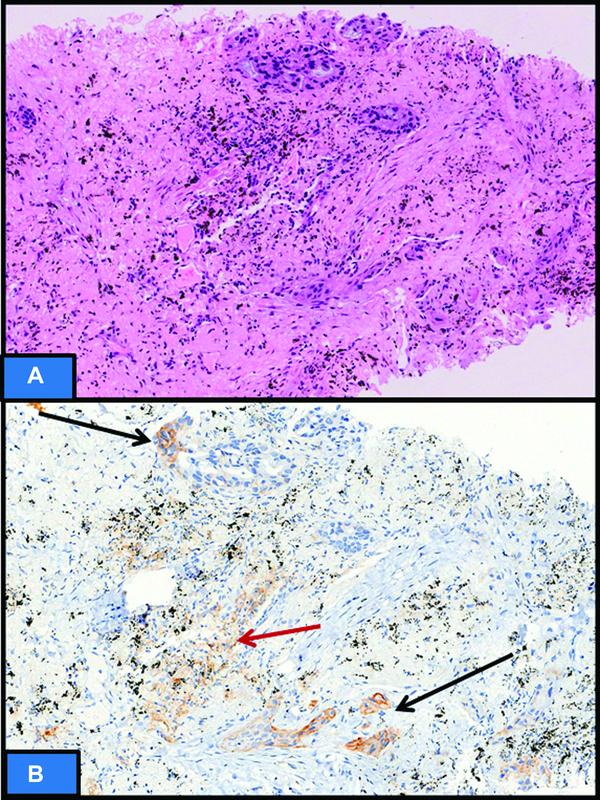
| Fig 1 : (A)Hematoxylin and eosin sections showing metastatic grade III, triple negative breast cancer in axillary lymph node with immune cells in tumor microenvironment (TME) (black arrow). (B and C) Programmed cell death protein ligand 1 (PD-L1) (SP142) immunohistochemistry showing immune cells staining (ICS) score of 5%. The ICS in TME only should be taken into consideration. PD-L1 staining in immune cells away from TME (red arrow) not in contact with tumor cells is not to be considered.

| Figure 2: (A) Hematoxylin and eosin sections showing primary breast invasive duct carcinoma, grade III, (triple negative breast cancer) with immune cells (TILS) in tumor microenvironment (arrow). (B) Programmed cell death protein ligand 1 (SP142) immunohistochemistry showing immune cells staining score of 20% (arrow). TILS: tumor infiltrating lymphocytes.

| Figure 3: (A) Hematoxylin and eosin section showing high-grade urothelial carcinoma of bladder with immune cells in tumor microenvironment (TME) (arrow). (B) Programmed cell death protein ligand 1 (SP142) immunohistochemistry showing immune cells staining score of 25% (black arrow). The immune cells away from TME (red arrow) will not be taken into consideration. No membranous staining seen in tumor cells.

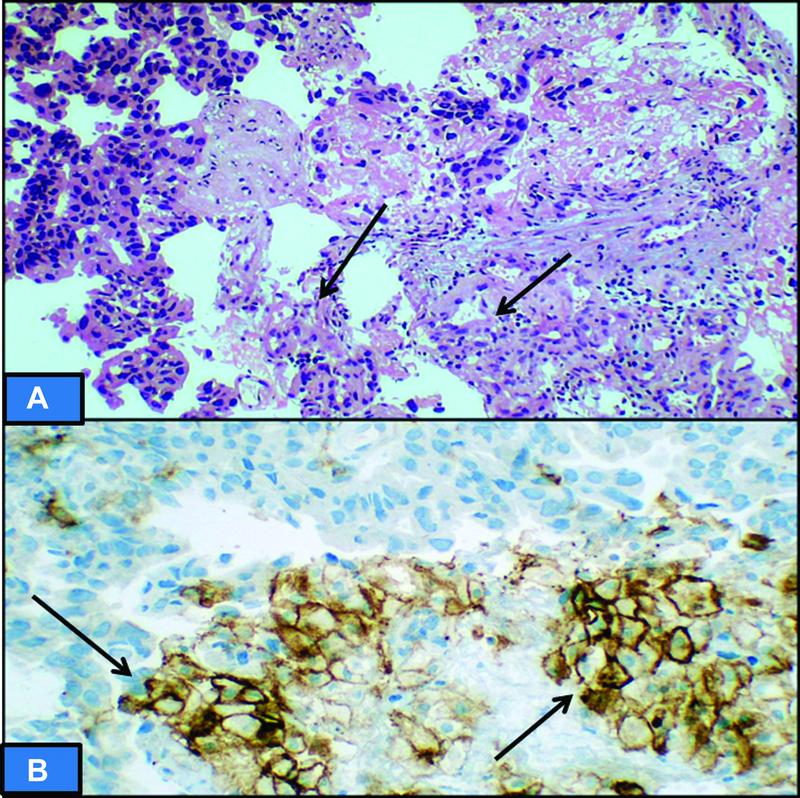
| Figure 4: (A) Hematoxylin and eosin section showing poorly differentiated adenocarcinoma of stomach. (B) Programmed cell death protein ligand 1 (PD-L1) immunohistochemistry showing CPS of 40. The high score is attributed to immune cells displaying PD-L1 staining, while very few tumor cells show membranous positivity (arrow).
| Figure 5: (A)Hematoxylin and eosin section showing non-small cell lung carcinoma in a tru-cut biopsy. (B) Distinct membranous programmed cell death protein ligand 1 immuno[removed]tumor proportion score) in 5%-of tumor cells of weak intensity (black arrow). Immune cells staining (red arrow) will not be taken into consideration.

| Figure 6: (A) Hematoxylin and eosin section showing non-small cell lung carcinoma in a tru-cut biopsy. (B) Distinct membranous programmed cell death protein ligand 1 immuno[removed]tumor proportion score) in 90% of tumor cells of moderate to strong intensity. Immune cells staining (red arrow) will not be taken into consideration.
| Fig 7: (A) Hematoxylin and eosin section showing tru-cut biopsy with non-small cell lung carcinoma with adjoining areas of histiocytes (arrow). (B) Distinct membranous programmed cell death protein ligand 1 immunoexpression in histiocytes that can be erroneously taken as positive tumor cell staining. Note that the size of nucleus of histiocytes is significantly smaller than the tumor nucleus (upper half unstained), and this serves as a clue to differentiate between the two.
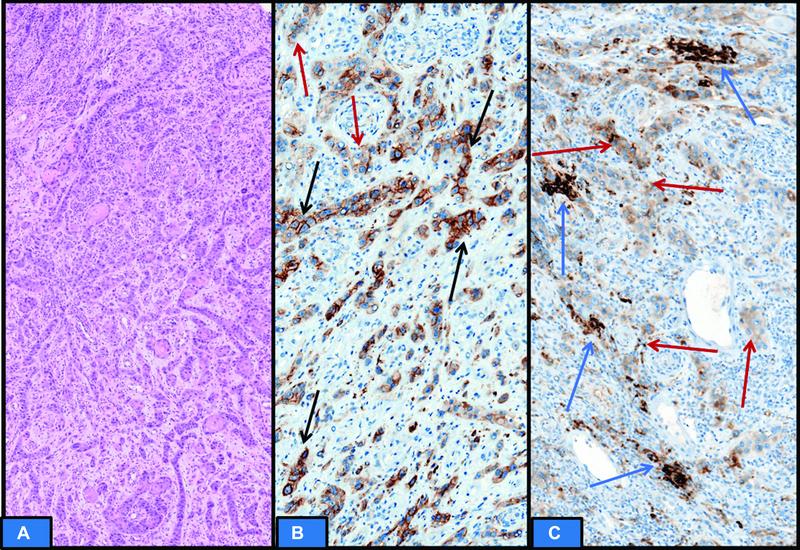
| fig 8: (A) Hematoxylin and eosin section showing recurrent locally advanced head and neck squamous cell carcinoma. (B and C) Programmed cell death protein ligand 1 immunoexpression is heterogenous. Few areas show partial and complete distinct membranous staining (black arrow) in tumor cells to be included in scoring. Significant tumor cells show cytoplasmic staining without membranous accentuation (red arrow), which is not to be counted in scoring. Immune cells staining is very less (blue arrow); however, it will be included in scoring. The final CPS was 25. cancer.
Tumor Proportion Score (TPS): It is scored as percentage of tumor cells showing distinct membranous staining. TPS is frequently utilized for metastatic non-small cell lung carcinoma (NSCLC). A potential misinterpretation can occur due to known membranous immunostaining of native pneumocytes or reactive histiocytes, which can be erroneously included in TPS ([Fig. 7]). Hence, correlation with histomorphology is prudent for accurate scoring.
TPS (%) = PD-L1 positive tumor cells x 100 Total tumor cells (PD-L1 positive + PD-L1 negative tumor cells)
Immune Cells Staining (ICS): It is scored as the percentage of tumor area that is occupied by PD-L1-stained immune cells of any intensity. ICS is commonly utilized for metastatic triple negative breast cancer and urothelial carcinoma. The scoring is done on immune cells only within tumor micro environment ([Fig. 3]). Areas of necrosis and granulation tissue should not be considered or sampled for assessment.
Combined Positive Score (CPS): It is scored as number of PD-L1-stained cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100. It is expressed in numbers and not in percentage, as it may exceed 100. CPS is frequently utilized for metastatic and recurrent head and neck squamous cell carcinoma as well as metastatic gastric/gastroesophageal adenocarcinoma ([Fig. 4]).
CPS= PD-L1 immunostained cells (tumor cells, lymphocytes, macrophages) × 100
Total viable tumor cells
Clinical Setting for PD-L1 IHC Testing
Companion Diagnostic Test: It is a prerequisite or mandatory test that provides information for the effective and safe use of an intended therapeutic drug. The various companion diagnostic PD-L1 assays with details are listed in [Table 1].
Complementary Diagnostic Tests: It is not a mandatory test before initiating the treatment with intended drug; however, it aids in the therapeutic decision. For example, Ventana SP142 PD-L1 assay is used as a complementary diagnostic test for intended treatment with Atezolizumab in previously treated NSCLC if TPS ≥ 50%-or IC score ≥ 10%.
|
Type of malignancy and affected organ |
Intended ICI and line of therapy |
Companion of diagnostic PD-L1 assay |
Type of scoring for PD-L1 immunoexpression |
Approving agency |
|---|---|---|---|---|
|
NSCLC (metastatic/UR stage III, NE for definite CT/RT) |
Pembrolizumab (anti-PD-1) 1st line monotherapy |
22C3 (Dako) |
TPS; ≥ 50% |
FDA |
|
NSCLC (metastatic/UR stage III, NE for definite CT/RT) |
Pembrolizumab (anti-PD-1) 1st line monotherapy |
SP263 (Ventana) |
TPS; ≥ 50% |
CE marked |
|
NSCLC (Metastatic) |
Pembrolizumab (anti-PD-1) 2nd line monotherapy |
22C3 (Dako) |
TPS; ≥ 01% |
FDA |
|
Urothelial carcinoma (LA/metastatic NE for CT) |
Atezolizumab 1st line monotherapy |
SP142 (Ventana) |
ICS; ≥ 05% |
FDA |
|
Urothelial carcinoma (LA/metastatic NE for CT) |
Pembrolizumab 1st line monotherapy |
22C3 (Dako) |
CPS; ≥ 10 |
FDA |
|
TNBC (recurrent LA/metastatic) |
Atezolizumab 1st line, in combination with nab-paclitaxel |
SP142 (Ventana) |
ICS; ≥ 01% |
FDA |
|
TNBC (recurrent LA/metastatic) |
Pembrolizumab (anti-PD-1) 1st line, in combination with nab-paclitaxel |
22C3 (Dako) |
CPS; ≥ 10 |
FDA |
|
Gastric/GEJ adenocarcinoma (recurrent LA/ metastatic) |
Pembrolizumab (anti-PD-1) 3rd line monotherapy |
22C3 (Dako) |
CPS; ≥ 01 |
FDA |
|
Cervical carcinoma (recurrent LA/ metastatic) |
Pembrolizumab (anti-PD-1) 2nd line monotherapy |
22C3 (Dako) |
CPS; ≥ 01 |
FDA |
|
Esophagus SCC (recurrent LA/metastatic) |
Pembrolizumab (anti-PD-1) 2nd line /3rd line monotherapy |
22C3 (Dako) |
CPS; ≥ 10 |
FDA |
|
HNSCC (metastatic/recurrent/UR) |
Pembrolizumab (anti-PD-1) 1st line monotherapy |
22C3 (Dako) |
CPS; ≥ 01 |
FDA |

| fig 9:On-slide positive and negative control (tonsillar tissue). The cryptic epithelium showing strong membranous positivity (red arrow), the surface epithelium is negative (black arrow), and the germinal centers show granular staining of moderate intensity (blue arrow). tuberous sclerosis.
Limitations of PD-L1 Testing and Future Perspectives
Although PD-L1 testing remains the most common predictive biomarker in current oncology practice, it is still an imperfect biomarker as some patients who are PD-L1 negative may still respond to ICI while those who are positive may not respond to ICI. The other challenge is intra- and intertumoral heterogeneity for PD-L1 immunoexpression that has implications in scoring and PD-L1 results. Moreover, with recent strategies to combine ICI with chemotherapy, it may further limit the precise significance of predictive utility of PD-L1 testing. A close collaboration between oncologist and pathologist is essential and further prospective large randomized trials are required to establish the precise role of biomarkers, especially PD-L1 for predicting response to ICI.[3] [4]
Conflict of Interest
None declared.
Source of Support
None declared.
References
-
Lantuejoul S, Sound-Tsao M, Cooper WA. et al. PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol 2020; 15 (04) 499-519
- Ionescu DN, Downes MR, Christofides A, Tsao MS. Harmonization of PD-L1 testing in oncology: a Canadian pathology perspective. Curr Oncol 2018; 25 (03) e209-e216
- Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunotherapy Cancer 2019; 278 (07) DOI: 10.1186/s40425-019-0768-9.
- Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J 2021; 23 (02) 39 DOI: 10.1208/s12248-021-00574-0.
- Tsao MS, Kerr KM, Kockx M. et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 2018; 13 (09) 1302-1311
Address for correspondence
Publication History
Article published online:
28 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 : (A)Hematoxylin and eosin sections showing metastatic grade III, triple negative breast cancer in axillary lymph node with immune cells in tumor microenvironment (TME) (black arrow). (B and C) Programmed cell death protein ligand 1 (PD-L1) (SP142) immunohistochemistry showing immune cells staining (ICS) score of 5%. The ICS in TME only should be taken into consideration. PD-L1 staining in immune cells away from TME (red arrow) not in contact with tumor cells is not to be considered.

| Figure 2: (A) Hematoxylin and eosin sections showing primary breast invasive duct carcinoma, grade III, (triple negative breast cancer) with immune cells (TILS) in tumor microenvironment (arrow). (B) Programmed cell death protein ligand 1 (SP142) immunohistochemistry showing immune cells staining score of 20% (arrow). TILS: tumor infiltrating lymphocytes.

| Figure 3: (A) Hematoxylin and eosin section showing high-grade urothelial carcinoma of bladder with immune cells in tumor microenvironment (TME) (arrow). (B) Programmed cell death protein ligand 1 (SP142) immunohistochemistry showing immune cells staining score of 25% (black arrow). The immune cells away from TME (red arrow) will not be taken into consideration. No membranous staining seen in tumor cells.

| Figure 4: (A) Hematoxylin and eosin section showing poorly differentiated adenocarcinoma of stomach. (B) Programmed cell death protein ligand 1 (PD-L1) immunohistochemistry showing CPS of 40. The high score is attributed to immune cells displaying PD-L1 staining, while very few tumor cells show membranous positivity (arrow).

| Figure 5: (A)Hematoxylin and eosin section showing non-small cell lung carcinoma in a tru-cut biopsy. (B) Distinct membranous programmed cell death protein ligand 1 immuno[removed]tumor proportion score) in 5% of tumor cells of weak intensity (black arrow). Immune cells staining (red arrow) will not be taken into consideration.

| Figure 6: (A) Hematoxylin and eosin section showing non-small cell lung carcinoma in a tru-cut biopsy. (B) Distinct membranous programmed cell death protein ligand 1 immuno[removed]tumor proportion score) in 90% of tumor cells of moderate to strong intensity. Immune cells staining (red arrow) will not be taken into consideration.

| Fig 7: (A) Hematoxylin and eosin section showing tru-cut biopsy with non-small cell lung carcinoma with adjoining areas of histiocytes (arrow). (B) Distinct membranous programmed cell death protein ligand 1 immunoexpression in histiocytes that can be erroneously taken as positive tumor cell staining. Note that the size of nucleus of histiocytes is significantly smaller than the tumor nucleus (upper half unstained), and this serves as a clue to differentiate between the two.

| fig 8: (A) Hematoxylin and eosin section showing recurrent locally advanced head and neck squamous cell carcinoma. (B and C) Programmed cell death protein ligand 1 immunoexpression is heterogenous. Few areas show partial and complete distinct membranous staining (black arrow) in tumor cells to be included in scoring. Significant tumor cells show cytoplasmic staining without membranous accentuation (red arrow), which is not to be counted in scoring. Immune cells staining is very less (blue arrow); however, it will be included in scoring. The final CPS was 25. cancer.

| fig 9:On-slide positive and negative control (tonsillar tissue). The cryptic epithelium showing strong membranous positivity (red arrow), the surface epithelium is negative (black arrow), and the germinal centers show granular staining of moderate intensity (blue arrow). tuberous sclerosis.
References
-
Lantuejoul S, Sound-Tsao M, Cooper WA. et al. PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol 2020; 15 (04) 499-519
- Ionescu DN, Downes MR, Christofides A, Tsao MS. Harmonization of PD-L1 testing in oncology: a Canadian pathology perspective. Curr Oncol 2018; 25 (03) e209-e216
- Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunotherapy Cancer 2019; 278 (07) DOI: 10.1186/s40425-019-0768-9.
- Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J 2021; 23 (02) 39 DOI: 10.1208/s12248-021-00574-0.
- Tsao MS, Kerr KM, Kockx M. et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 2018; 13 (09) 1302-1311


 PDF
PDF  Views
Views  Share
Share

