Papillary squamotransitional cell carcinoma of the uterine cervix: A histomorphological and immunohistochemical study of nine cases
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(02): 66-71
DOI: DOI: 10.4103/0971-5851.116177
Abstract
Background: Papillary squamotransitional cell carcinoma (PSCC) is a distinctive subcategory of squamous cell carcinoma of the uterine cervix. It has a propensity for local recurrence and late metastasis. Histologically, it can be misinterpreted as transitional cell carcinoma, or other papillary lesions of the cervix including squamous papilloma, verrucous carcinoma or cervical intraepithelial neoplasia grade 3 with papillary configuration. Materials and Methods: Nine cases of PSCC of the uterine cervix were diagnosed on a cervical biopsy specimen on routine hematoxylin and eosin (H and E) stained sections. Their clinic-morphological features were analyzed. The cases were further evaluated immunohistochemically by cytokeratin 7 (CK7), cytokeratin 20 (CK20), p53 and Ki-67. Results: The patients ranged in age from 35 years to 75 years; with abnormal uterine bleeding being the most common clinical presentation. All the cases showed papillary architecture with fibrovascular cores lined by multilayered atypical epithelium. Three cell types were observed: Clear, intermediate and basaloid. Stromal invasion was seen in five cases, whereas in the remaining four cases, the biopsy specimen was too superficial to definitely assess invasion. Immunohistochemically, eight cases were CK7 + /CK20 - and one case was CK7 - /CK20 - . All nine cases showed nuclear accumulation of mutant p53. Moderate and high proliferative activity was observed in two and seven cases, respectively. Five of patients for whom follow-up information was available underwent radical hysterectomy and two of them were disease free 18 months following treatment. Conclusion: PSCC of the uterine cervix are a clinicomorphologically distinct group of cervical lesions that display a morphologic spectrum. They are potentially aggressive malignant tumors that should be distinguished from transitional cell carcinoma and other papillary lesions of the uterine cervix.
Keywords
Cervix - cytokeratin 7/cytokeratin 20 - Ki-67 - p53 - papillary squamotransitional cell carcinomaPublication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Papillary squamotransitional cell carcinoma (PSCC) is a distinctive subcategory of squamous cell carcinoma of the uterine cervix. It has a propensity for local recurrence and late metastasis. Histologically, it can be misinterpreted as transitional cell carcinoma, or other papillary lesions of the cervix including squamous papilloma, verrucous carcinoma or cervical intraepithelial neoplasia grade 3 with papillary configuration.
Materials and Methods:
Nine cases of PSCC of the uterine cervix were diagnosed on a cervical biopsy specimen on routine hematoxylin and eosin (H and E) stained sections. Their clinic-morphological features were analyzed. The cases were further evaluated immunohistochemically by cytokeratin 7 (CK7), cytokeratin 20 (CK20), p53 and Ki-67.
Results:
The patients ranged in age from 35 years to 75 years; with abnormal uterine bleeding being the most common clinical presentation. All the cases showed papillary architecture with fibrovascular cores lined by multilayered atypical epithelium. Three cell types were observed: Clear, intermediate and basaloid. Stromal invasion was seen in five cases, whereas in the remaining four cases, the biopsy specimen was too superficial to definitely assess invasion. Immunohistochemically, eight cases were CK7+/CK20– and one case was CK7–/CK20–. All nine cases showed nuclear accumulation of mutant p53. Moderate and high proliferative activity was observed in two and seven cases, respectively. Five of patients for whom follow-up information was available underwent radical hysterectomy and two of them were disease free 18 months following treatment.
Conclusion:
PSCC of the uterine cervix are a clinicomorphologically distinct group of cervical lesions that display a morphologic spectrum. They are potentially aggressive malignant tumors that should be distinguished from transitional cell carcinoma and other papillary lesions of the uterine cervix.
INTRODUCTION
Papillary squamotransitional cell carcinoma (PSCC) of the uterine cervix is a distinct clinico-pathological subtype of cervical cancer.[1] These tumors have been described at other sites of the female genital tract like the ovary and vagina,[2,3] and were designated as “transitional cell carcinoma” by Albores-Saavedra and Young.[4] Recently, Randall et al. re-designated these tumors as “papillary squamous cell carcinoma.”[5] PSCC can show a morphological spectrum from pure squamous carcinoma to purely transitional appearance.[1] The tumor has a distinctive papillary growth pattern, tends to invade deeply and has a propensity for late metastasis and late recurrence.[6] Since, it is not a very well-known entity and has a different behavior, it should be segregated from transitional cell carcinoma and other papillary lesions of the cervix, including condyloma, squamous papilloma, verrucous carcinoma, and cervical intraepithelial neoplasia grade 3 (CIN3) with papillary configuration.[6,7] In this report, we analyze the histomorphological and immunohistochemical features of nine cases of PSCC of the uterine cervix, along with review of relevant literature.
MATERIALS AND METHODS
Nine cases of PSCC of the uterine cervix were diagnosed on a cervical biopsy specimens received in the department of Pathology over a period of 3 years, on routine hematoxylin and eosin stained sections. The histomorphological features of each case like papillae formation, fibrovascular cores, cell types, the presence of atypia/mitosis, stromal invasion and infiltration; were noted and evaluated independently by two pathologists and the results compared. Immunohistochemistry was performed on formalin fixed paraffin sections of each tumor using manual polymer detection system with heat induced epitope retrieval. The following pre-diluted ready to use primary antibodies were used - cytokeratin 7 (CK7) (clone OV-TL 12/30; Dako), cytokeratin 20 (CK20) (clone KS-20.8; Dako), p53 (clone DO-7; Dako) and Ki-67 (clone MIB-1; Dako). Immunoreactivity for CK7 and CK20 was quantitatively assessed in each case by estimating the percentage of cells showing membrane staining, and scoring the intensity of staining as 0, 1+, 2 + or 3+. If at least 5% of the cells displayed at least 1+ intensity, the case was considered positive. The reaction for p53 was recorded as either positive or negative; whereas the nuclear staining for Ki-67 was graded as minimal ( < 10% of cells), moderate (10-50% of cells) and high (>50% of cells). The clinical details were retrieved from the case files.
RESULTS
Clinical findings
The mean age of the study population was 55 years with a range between 35 years and 75 years. The presenting symptoms included post-coital bleeding (one case), metrorrhagia (one case), postmenopausal bleeding (five cases) and abnormal Papanicolaou (Pap) smear (two cases). Other associated systemic complaints were weight loss, fever, and pelvic pain. None of the patients had a history of prior, recurrent or subsequent transitional cell carcinoma of the urinary tract. Local examination in almost all the cases revealed bulky friable polypoidal masses involving the cervix.
Histopathological findings
[Table 1]: All the nine cases on histopathological examination showed the presence of papillae consisting of fibrovascular cores lined by multilayered atypical epithelial cells resembling transitional type epithelium [Figure [Figure1a1a and andb].b]. However, one case showed the presence of micropapillae and no fibrovascular cores [Figure 1c]. Three cell types were identified: Intermediate, basaloid, and clear cells. Six cases were comprised predominantly of intermediate cells, one case showed clear cells and the remaining two cases showed both intermediate and basaloid cells. Out of these two cases, one case was comprised predominantly of basaloid cells with only the peripheral portion of the papillae lined by intermediate cells [Figure 1e]. The intermediate cells had a moderate amount of eosinophilic cytoplasm, hyperchromatic to vesicular nuclei with prominent nucleoli. The clear cells were comprised of abundant clear cytoplasm, vesicular nucleus with prominent nucleoli [Figure 1d]. The basaloid cells had scanty cytoplasm, large hyperchromatic nucleus with high nucleo-cytoplasmic ratio. The cells showed features of moderate to severe atypia like pleomorphism, anisonucleosis, hyperchromatism and increased nucleo-cytoplasmic ratio. Multinucleated giant cells were also seen in a few cases. Mitoses ranged from 1-2 (four cases) to 6-7 (two cases) per high power field (HPF). Remaining three cases showed 3-4 mitotic figures/HPF. Frequent abnormal mitotic figures were noted in a few cases [Figure 1f]. Intracellular keratinization was seen in five cases, out of which two cases showed keratin pearl formation as well [Figure 1g]. Five cases showed clear-cut evidence of stromal invasion in the form of tiny nests and bulbous projections surrounded by edema and chronic inflammatory infiltrate [Figure 1h]. In the remaining four cases, the biopsy specimen was too superficial to definitely assess invasion. Chronic inflammatory infiltrate in the stroma was seen in all cases, with one case demonstrating stromal infiltrate comprising of neutrophils and eosinophils [Figure 1i]. Cytological changes suggestive of human papillomavirus (HPV) infection, like koilocytosis, were seen in three cases [Figure 2]. Two cases showed foci of tumor necrosis.
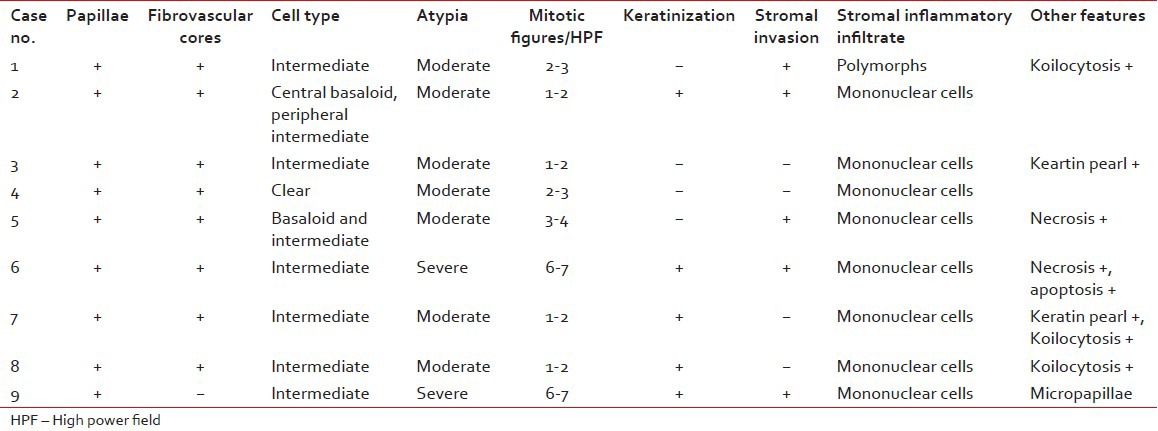
Table 1
Histomorphological features of nine cases of papillary squamotransitional cell carcinoma
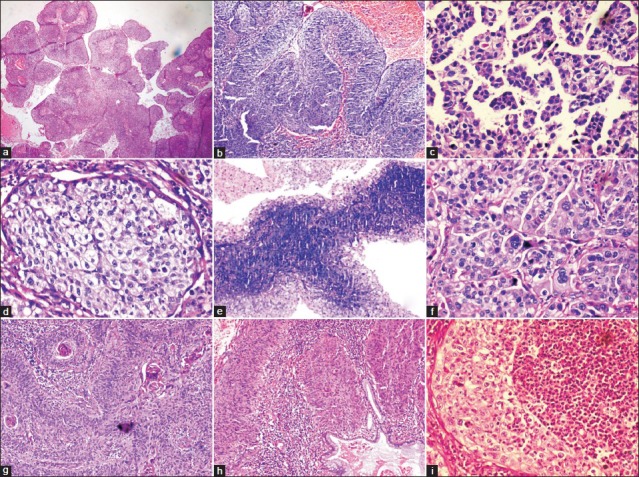
| Fig. 1 (a) Prominent papillary architecture (H and E, ×40); (b) Typical papillary squamotransitional cell carcinoma (H and E, ×100); (c) Micropapillary architecture (H and E, ×400); (d) Clear cell change (H and E, ×400); (e) Papillae lined by central basaloid and peripheral intermediate cells (H and E, ×100); (f) Prominent pleomorphism, atypical mitotic figures and multinucleated giant cells (H and E, ×400); (g) Intracellular keratinisation and keratin pearl formation (H and E, ×100); (h) Invasive PSCC with endocervical gland involvement (H and E, ×100); (i) PSCC with polymorphonuclear inflammatory infiltrate (H and E, ×400)
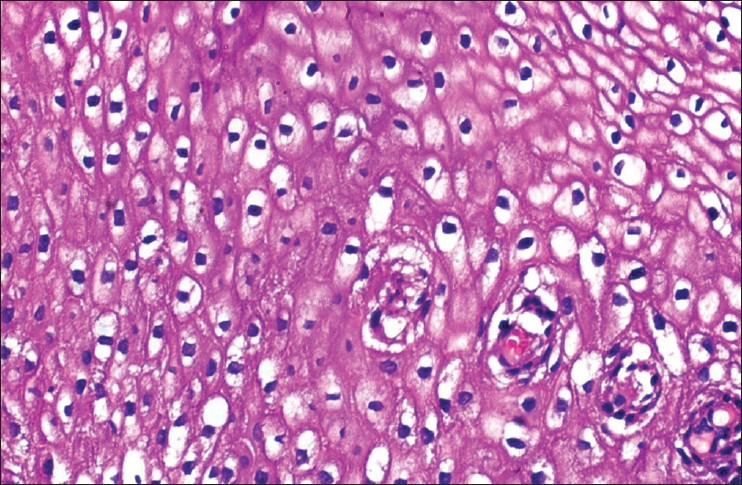
| Fig. 2 Koilocytosis within the the tumor (H and E, ×400)
Immunohistochemical findings
Table 2 - Eight cases showed positive immunostaining for CK7 and negative immunostaining for CK20, whereas one case was negative for both CK7 and CK20. All nine cases showed nuclear accumulation of mutant p53. Moderate and high proliferative activity (Ki-67 immunostaining) was observed in two and seven cases respectively [Figure 3].
Table 2
Immunohistochemical profile of nine cases of papillary squamotransitional cell carcinoma
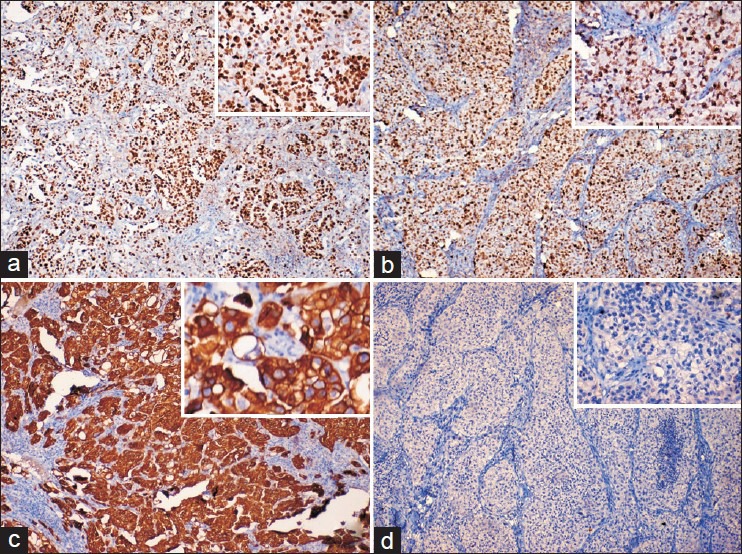
| Fig. 3 Immunohistochemical stains revealing the tumor cells showing (a) Positive nuclear staining for Ki-67 (×100; inset [×400]); (b) Nuclear accumulation of mutant p53 (×100; inset [×400]); (c) Strong cytoplasmic reactivity for CK7 (×100; inset [×400]); (d) Negative reactivity for cytokeratin 20 (×100; inset [×400])
Treatment
Five cases underwent further work-up whereas four cases were lost following the initial biopsy. Of these five cases, two had stage IB1, two had stage IIB and one patient had stage IIIA disease. All these patients underwent radical hysterectomy and were referred to a tertiary care centre for further treatment. Follow-up information was available for three of these patients, one of whom had died of the disease and the other two were disease free 18 months following the treatment. The patient who died of the disease presented with tumor spread to bladder wall with occlusion of ureteric orifice resulting in hydronephrosis and consequent renal failure; 14 months after the initial diagnosis.
DISCUSSION
Papillary carcinoma of the uterine cervix with transitional or squamous differentiation are rare tumors that often resemble transitional cell carcinoma of the urinary tract.[8] These tumors were initially identified by Marsh in 1952.[9] In his study, three out of 31 papillary tumors of the cervix turned out to be malignant. In 1958, Kazal reported 20 cases of squamous cell papillomas of the cervix, of which two showed transformation to squamous cell carcinoma.[10] These tumors were characterized as papillary squamous cell carcinoma by Randall et al. in 1986.[5] It remains unclear whether papillary carcinomas of the cervix represent two clinicopathologically distinct group of tumors (squamous and transitional) or if they reflect morphologic continuum within a single clinically homogeneous entity. The Armed Forces Institute of Pathology fascicle uses the terms “squamous” and “transitional” interchangeably to refer to these papillary carcinomas of the cervix.[8,11] A comprehensive study by Koenig et al. of 32 cases of papillary cervical carcinomas reported that tumors can be divided into three groups based on their histologic appearance: Predominantly squamous, predominantly transitional and mixed squamous and transitional.[8]
Papillary squamous cell carcinoma has also been reported at other sites like the vagina and endometrium; and appears to have similar histomorphological features, immunohistochemical profile and association with HPV as PSCC and squamous cell carcinoma of the cervix.[12,13]
The clinical presentation of most of our cases was similar to other studies, i.e., elderly lady presenting with postmenopausal bleeding or abnormal pap smear.[5,8] Only two patients were in the younger age group who presented with postcoital bleeding and metrorrhagia, respectively.
PSCC's have a complex architecture enabling only a superficial biopsy. On biopsy, the histological appearances often suggest a superficial lesion, but the diagnosis has to be made on architecture, even if invasion is not seen and atypia is moderate.[1] Most of our cases showed papillary architecture with prominent fibrovascular cores, as reported by other authors as well.[1,5] Only one case showed micropapillae without any fibrovascular cores. To the best of our knowledge, micropapillary architecture in PSCC has not yet been reported in the literature.
Invasion may be difficult to demonstrate histologically unless deep biopsies are obtained. A high index of suspicion and an awareness of this entity are required to make the diagnosis. The assessment of tumor depth in excision specimen is a difficult task because of complex surface papillary architecture of these lesions. This can result in incorrect staging which can affect the patient's treatment and prognosis.[1] 55% of our cases showed evidence of stromal invasion. The results were compared to studies by Koenig et al.[1] and Kokka et al.[8] in which 56% and 66% of the cases showed stromal infiltration by the tumor, respectively.
Three cell types were observed in the present study. 66% of the cases were comprised predominantly of intermediate cells. 22% cases showed both intermediate and basaloid cells, whereas 11% showed only clear cells. Presence of intermediate and basaloid cells in PSCC has also been reported in a study from Uganda.[14] Clear cell change has also been reported in two out of 16 cases of PSCC by Koenig et al.[8]
Most of our cases showed evidence of keratinisation with keratin pearl formation evident in two cases. One out of three cases of PSCC studied by Kokka et al. also showed keratin pearl formation.[1]
The differential diagnoses include CIN3 with papillary configuration, condyloma, squamous papilloma;[1] or transitional cell carcinoma,[8] endometroid adenocarcinoma, papillary serous carcinoma and well-differentiated villoglandular (papillary) adenocarcinoma.[15]
Papillary immature metaplasia (PIM) is a distinctive exophytic lesion of the uterine cervix which shares some histomorphologic features with PSCC. PIM shows characteristic filiform papillae, with immature squamous epithelium retaining the mucus cells on the surface of the papillae. The individual cells show mild nuclear atypia and infrequent mitotic figures. In contrast, PSCC shows narrower and longer fibrovascular cores with more diffuse and marked cytological atypia and pleomorphism. Ki-67 labeling index and p53 expression are much higher in PSCC as compared to PIM.[16]
Koenig et al. suggested that the vast majority of PSCC's display the cytokeratin profile of squamous cell carcinoma of the cervix (i.e., CK7+/CK20–) in contrast to transitional cell carcinoma of the urinary bladder (CK7+/CK20+). One of the case of squamous cell carcinoma of the urinary bladder in their study also lacked CK20 expression suggesting that loss of CK20 immunoreactivity parallels the loss of classic transitional features, and development of squamous differentiation in transitional cells, a finding also noted by Moll et al.[8,17] Lininger et al. evaluated CK7/20 profile of seven cases of transitional cell carcinoma of the endometrium out of which all the cases were CK20– and five were CK7+.[18] Almost all the cases in the present study were CK7+/CK20–, except a single case which was CK7-/CK20–. In a study by Drew et al., eight cases were immunoreactive for p53 and uroplakin III was negative in 14 cases. The above mentioned immunohistochemical profile suggests that PSCC is a variant of squamous cell carcinoma with retention of a Mullerian immunoprofile, although showing morphological features of urothelial differentiation.[1,19]
The mechanism underlying the development of PSCC is still under investigation, however HPV has been implicated as one of the etiologic agents.[1,8,13,20] Koenig et al. noted that HPV like changes in six of their PSCCs, supporting the notion of a progression through an in situ phase and a possible etiologic role for HPV.[8] In the present study also, three cases showed cytological evidence of HPV infection.
The molecular biology of tumor suppressor gene p53 has been thoroughly elucidated in the development of cervical carcinoma. More than 95% of cervical carcinomas are associated with HPV infection. The virus produces the E6 oncoprotein, which binds to p53 and facilitates its degradation. This leads to a state of p53 dysfunction similar to p53 mutation in other tumors. In a study by Mirhashemi et al., three out of twelve cases of PSCC stained positive for p53.[7] In the present study, all the nine cases showed p53 positivity. This is significantly higher than what is observed for squamous cell carcinoma in general. This can be attributed to the small sample size and the rarity of this entity.
Finally, the growth fraction of PSCC was assessed using the proliferation marker Ki-67. Ki-67 antibody (MIB-1) reacts with nuclear non-histone proteins and reflects the growth fraction of a tumor. In a study by Mirhashemi et al., eight out of twelve PSCC's displayed high proliferative activity.[7] Similar results were obtained in the present study, with two cases showing moderate and seven cases showing high proliferative activity.
PSCC responds to the same therapeutic approaches as does non-papillary squamous cancer, therefore the treatment should depend on the clinical stage and the patient's condition.[1] Koenig et al. reported longer survival in one of their patients with advanced disease treated by chemotherapy. Hence, the role of chemotherapy in the treatment of these tumors needs to be further explored.[8]
Several reports show that PSCC's have a tendency for late recurrences and late metastasis.[5,8] Koenig et al. reported a vaginal recurrence 12 years after the initial diagnosis.[8] Similar findings were also noted by Randall et al. in two of their nine patients.[5] PSCC is a not a very well-known entity. Further investigations into the biologic and genetic nature, and clinicopathologic profile of PSCC with a larger case series are warranted which may help predict its clinical course and further direct clinical therapies.[7]
Although, the cytokeratin profile of PSCC is more or less similar to conventional squamous cell carcinoma; the histomorphological observations in combination with immunohistochemistry and prognostic markers like Ki-67 and p53, will help us to distinguish PSCC from transitional cell carcinoma and other borderline papillary lesions of the uterine cervix; and predict its biological behavior as well.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 (a) Prominent papillary architecture (H and E, ×40); (b) Typical papillary squamotransitional cell carcinoma (H and E, ×100); (c) Micropapillary architecture (H and E, ×400); (d) Clear cell change (H and E, ×400); (e) Papillae lined by central basaloid and peripheral intermediate cells (H and E, ×100); (f) Prominent pleomorphism, atypical mitotic figures and multinucleated giant cells (H and E, ×400); (g) Intracellular keratinisation and keratin pearl formation (H and E, ×100); (h) Invasive PSCC with endocervical gland involvement (H and E, ×100); (i) PSCC with polymorphonuclear inflammatory infiltrate (H and E, ×400)

| Fig. 2 Koilocytosis within the the tumor (H and E, ×400)

| Fig. 3 Immunohistochemical stains revealing the tumor cells showing (a) Positive nuclear staining for Ki-67 (×100; inset [×400]); (b) Nuclear accumulation of mutant p53 (×100; inset [×400]); (c) Strong cytoplasmic reactivity for CK7 (×100; inset [×400]); (d) Negative reactivity for cytokeratin 20 (×100; inset [×400])


 PDF
PDF  Views
Views  Share
Share

