Pancreatitis in Acute Promyelocytic Leukemia: Drug induced or Differentiation Syndrome
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 371-373
DOI: DOI: 10.4103/ijmpo.ijmpo_36_16
Abstract
Acute promyelocytic leukemia (APL) constitutes about 15% of all acute myeloid leukemia patients and can now be treated even without any chemotherapy, with all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO). Acute pancreatitis (AP) is a rare adverse event in APL, which is primarily reported to be secondary to hypertriglyceridemia. Here, we have reported AP developed in a patient of APL, during induction with ATRA and ATO, but it was not associated with hypertriglyceridemia. Rather, it was associated with respiratory distress and weight gain, coincidental leukocytosis, bilateral pleural effusion, and edematous pancreatitis without any necrosis. Hence, AP in this case is diagnosed to be a manifestation of differentiation syndrome, and it responded to steroid.
Keywords
Acute promyelocytic leukemia - all-trans-retinoic acid - arsenic - differentiation syndrome - pancreatitisPublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Acute promyelocytic leukemia (APL) constitutes about 15% of all acute myeloid leukemia patients and can now be treated even without any chemotherapy, with all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO). Acute pancreatitis (AP) is a rare adverse event in APL, which is primarily reported to be secondary to hypertriglyceridemia. Here, we have reported AP developed in a patient of APL, during induction with ATRA and ATO, but it was not associated with hypertriglyceridemia. Rather, it was associated with respiratory distress and weight gain, coincidental leukocytosis, bilateral pleural effusion, and edematous pancreatitis without any necrosis. Hence, AP in this case is diagnosed to be a manifestation of differentiation syndrome, and it responded to steroid.
Introduction
Acute promyelocytic leukemia (APL) constitutes about 15% of all acute myeloid leukemia (AML) patients[1] and its treatments differ from other AML. With the introduction of all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO), APL can now be treated even without any chemotherapy. Careful monitoring and successful treatment of these complications may result in complete remission in about 85% of patients.[2] Reported adverse effects of ATRA vary from dry skin to retinoic acid syndrome.[3] Acute pancreatitis (AP) is a rare adverse event which is primarily reported to be secondary to hypertriglyceridemia. Here, we are presenting an interesting case of APL-developed AP as a manifestation of differentiation syndrome.
An 18-year-old female patient presented to us with bleeding from gum and multiple purpuric spot all over the body for 7 days, along with mild low-grade fever. At presentation, she was pale, with multiple purpura over the body, presence of wet purpura in buccal mucosa, no icterus, and mild hepatosplenomegaly, without any lymphadenopathy. Her initial blood report revealed total lymphocyte count of 7.2 × 109/L with the presence of abnormal promyelocytes. Bone marrow immunophenotyping and cytogenetics study was suggestive of APL, and polymerase chain reaction for PML-RARA was positive. Hence, diagnosis of APL, Sanz intermediate risk, was made. The patient was started on ATRA (45 mg/m2) and ATO (0.15 mg/Kg) according to the institutional protocol and was monitored for disseminated intravascular coagulation (DIC) as well as for differentiation syndrome. The initial DIC parameters improved within 5 days of starting ATRA; the patient started improving with cessation of bleeding manifestation and subsidence of fever. On day 12 of ATRA therapy, the patient complained of respiratory distress with vague abdominal pain. On examination, there was no icterus, mild abdominal tenderness in epigastrium not radiating to anywhere, bowel sound was sluggish, and there was no rigidity or rebound tenderness. Chest was clear on auscultation. Within next 2 days, the pain increased, along with radiation to back, with abdominal guarding and absent bowel sound. There was diminished breath sound in bilateral lower zone of the chest with crepitation in basal region. She did not have any fever, bleeding, or jaundice, but regular weight monitoring revealed weight gain of about 6 kg in the past 3 days.
The complete hemogram revealed an increase in total lymphocyte count of 26.5 × 109/L to 62.5 × 109/L from day 12 to day 15 of ATRA therapy. Furthermore, the biochemical parameters revealed an increase in serum lipase, amylase level (peak value 1229 IU/dl and 940 IU/dl, respectively), and mild increase in liver enzymes (serum glutamyl pyruvate transaminase and serum glutamic oxaloacetic transaminase are 346 and 380 IU/dl, respectively). The lipid profile, electrolytes, and calcium level were within normal range (calcium: 9.2 mg/dl, triglyceride: 194 mg/dl) [Figure 1]. The prothrombin time, activated partial thromboplastin time, fibrinogen, and D-dimer were normal. The biochemical parameters were suggestive of AP, and contrast-enhanced computed tomography (CECT) abdomen revealed bulky edematous pancreatitis without any area of necrosis and bilateral pleural effusion [Figures [Figures22 and and33].
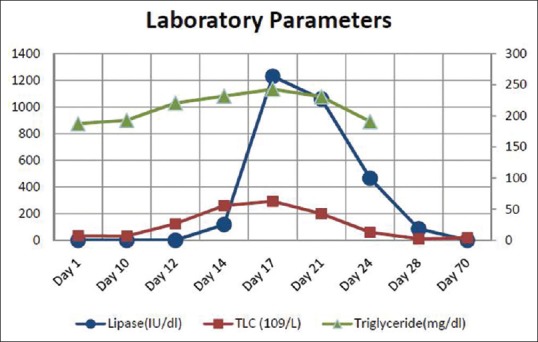
| Figure 1:Change in total leukocyte count, lipase and triglyceride level during the development of acute pancreatitis

| Figure 2:Straight X-ray abdomen in erect posture showing colon cutoff sign
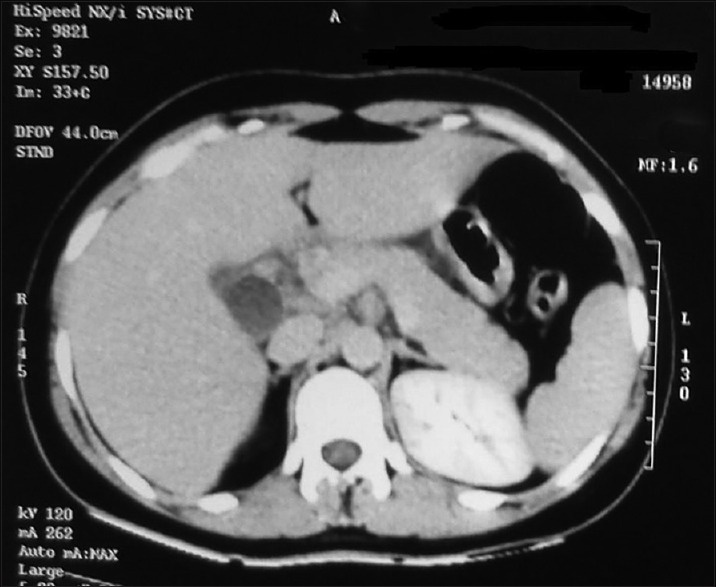
| Figure 3:Contrast-enhanced computed tomography abdomen showing bulky edematous pancreatitis
She was started on empirical broad-spectrum antibiotics after sending blood culture and on dexamethasone 10 mg twice daily. The ATRA and arsenic were kept withheld until symptom subsides, and she improved in next 8 days. The ATRA was started on a lower dose (25 mg/m2) and she achieved complete remission on day 70 of ATRA therapy.
Discussion
AP in APL is a rare complication. Very few case reports are available which depicts the development of AP in APL. The exact etiopathogenesis of AP sometimes remains unknown. The two most common causes of AP in general population, namely, alcohol and common bile duct obstruction by gallstone are not applicable here.
Drug-induced AP accounts 1.5%–2% of all AP.[4] Both ATRA and ATO can rarely cause AP. The common mechanism of AP with ATRA is due to the development of hypertriglyceridemia. ATRA-related AP has been previously reported by different authors.[5,6] In all these cases, patients had hypertriglyceridemia. However, few case reports also described development of AP without hypertriglyceridemia.[7] In our patient, the development of AP was not associated with hypertriglyceridemia.
The development of AP as a presentation of acute arsenic toxicity is far less common and reported only in four case reports previously.[8,9,10,11] In all of them, higher dose of ATO or oral administration of ATO is used, and they were associated with manifestation of acute arsenic toxicity such as diarrhea, abdominal pain, and jaundice. Monitoring of urinary arsenic excretion can help. Values >50–100 μg/24 h suggests arsenic intoxication.[8] A fatal dose range lies between 100 and 300 mg although smaller doses may also be life-threatening.[12] Our patient received arsenic at a dose 0.15 mg/kg daily for about 14 days before AP.
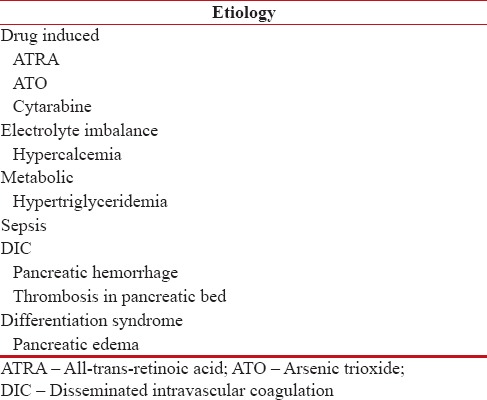
The possible causes of AP in APL are delineated in Table 1. Hypercalcemia was ruled out in our patient by biochemical tests. Probability of the development of microvascular thrombosis in pancreatic bed due to DIC was also unlikely because the patient already had resolution of all bleeding manifestation and DIC markers were normal. Furthermore, DIC after 2 weeks of ATRA therapy is very unlikely in APL patients.
Table 1
Causes of pancreatitis in acute promyelocytic leukemia
| The patient had initial symptoms of respiratory distress before overt AP. Furthermore, the patient had significant weight gain during the development of AP. The total lymphocyte count was also raised during the period. The patient also developed bilateral pleural effusion concomitantly. The CECT showed edematous bulky pancreatitis without any necrosis. The patient's symptoms subsided with stoppage of therapy and dexamethasone. All these lead to a diagnosis of differentiation syndrome which manifested in the form of AP. The differentiation syndrome occurs due to release of cytokines from granules of abnormal promyelocytes while they differentiate which lead to endothelial leakage. This leads to widespread edema, including pulmonary edema and weight gain. The pancreatic edema may cause occlusion of pancreatic duct leading to the development of AP. The manifestation of differentiation syndrome in the form of AP is not mentioned in literature before. To the best of our knowledge, this is first case report, where APL patient developed AP as a manifestation of differentiation syndrome due to ATRA and ATO therapy.
AP is a rare complication of APL during chemotherapy. Multiple etiological factors can cause AP in these patients, of which drug-induced AP is the most common cause. AP as a form of differentiation syndrome is rare and can occur due to edematous pancreatic duct leading to obstruction. Careful monitoring and exclusion of all other causes needed for the management of such lethal complication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Warrell RP Jr., de The H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med 1993;329:17-89.
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 1998;339:1341-8.
- Hatake K, Uwai M, Ohtsuki T, Tomizuka H, Izumi T, Yoshida M, et al. Rare but important adverse effects of all-trans retinoic acid in acute promyelocytic leukemia and their management. Int J Hematol 1997;66:13-9.
- Koshy GK, Kumar S, Hertan HI. All-trans-retinoic acid (tretinoin) induced fatal acute pancreatitis. Am J Gastroenterol 2003;98 (Suppl S9):S164.
- Abou Chacra L, Ghosn M, Ghayad E, Honein K. A case of pancreatitis associated with all-trans-retinoic acid therapy in acute promyelocytic leukemia. Hematol J 2001;2:406-7.
- Izumi T, Hatake K, Miura Y. Acute promyelocytic leukemia. N Engl J Med 1994;330:141.
- Teng HW, Bai LY, Chao TC, Wang WS, Chen PM. Acute pancreatitis during all-trans-retinoic acid treatment for acute promyelocytic leukemia in a patient without overt hypertriglyceridemia. Jpn J Clin Oncol 2005;35:94-6.
- Zaloga GP, Deal J, Spurling T, Richter J, Chernow B. Unusual manifestations of arsenic intoxication. Am J Med Sci 1985;289:210-4.
- Yamano T, Yokote T, Akioka T, Hara S, Oka T, Tsuji M, et al. Acute pancreatitis during the treatment of relapsed acute promyelocytic leukemia with As2O3. Rinsho Ketsueki 2006;47:23-5.
- Hantson P, Haufroid V, Buchet JP, Mahieu P. Acute arsenic poisoning treated by intravenous dimercaptosuccinic acid (DMSA) and combined extrarenal epuration techniques. J Toxicol Clin Toxicol 2003;41:1-6.
- Connelly S, Zancosky K, Farah K. Arsenic-induced pancreatitis. Case Rep Gastrointest Med 2011;2011:758947.
- Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J 2003;79:391-6.

| Figure 1:Change in total leukocyte count, lipase and triglyceride level during the development of acute pancreatitis

| Figure 2:Straight X-ray abdomen in erect posture showing colon cutoff sign

| Figure 3:Contrast-enhanced computed tomography abdomen showing bulky edematous pancreatitis
References
- Warrell RP Jr., de The H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med 1993;329:17-89.
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 1998;339:1341-8.
- Hatake K, Uwai M, Ohtsuki T, Tomizuka H, Izumi T, Yoshida M, et al. Rare but important adverse effects of all-trans retinoic acid in acute promyelocytic leukemia and their management. Int J Hematol 1997;66:13-9.
- Koshy GK, Kumar S, Hertan HI. All-trans-retinoic acid (tretinoin) induced fatal acute pancreatitis. Am J Gastroenterol 2003;98 (Suppl S9):S164.
- Abou Chacra L, Ghosn M, Ghayad E, Honein K. A case of pancreatitis associated with all-trans-retinoic acid therapy in acute promyelocytic leukemia. Hematol J 2001;2:406-7.
- Izumi T, Hatake K, Miura Y. Acute promyelocytic leukemia. N Engl J Med 1994;330:141.
- Teng HW, Bai LY, Chao TC, Wang WS, Chen PM. Acute pancreatitis during all-trans-retinoic acid treatment for acute promyelocytic leukemia in a patient without overt hypertriglyceridemia. Jpn J Clin Oncol 2005;35:94-6.
- Zaloga GP, Deal J, Spurling T, Richter J, Chernow B. Unusual manifestations of arsenic intoxication. Am J Med Sci 1985;289:210-4.
- Yamano T, Yokote T, Akioka T, Hara S, Oka T, Tsuji M, et al. Acute pancreatitis during the treatment of relapsed acute promyelocytic leukemia with As2O3. Rinsho Ketsueki 2006;47:23-5.
- Hantson P, Haufroid V, Buchet JP, Mahieu P. Acute arsenic poisoning treated by intravenous dimercaptosuccinic acid (DMSA) and combined extrarenal epuration techniques. J Toxicol Clin Toxicol 2003;41:1-6.
- Connelly S, Zancosky K, Farah K. Arsenic-induced pancreatitis. Case Rep Gastrointest Med 2011;2011:758947.
- Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J 2003;79:391-6.


 PDF
PDF  Views
Views  Share
Share

