Palmar-plantar erythrodysesthesia: An uncommon adverse effect of everolimus
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(02): 116-118
DOI: DOI: 10.4103/0971-5851.180143
Abstract
Mammalian target of rapamycin inhibitor everolimus is a novel agent used in endocrine therapy resistant hormone receptor positive metastatic breast cancer. Its use has been associated with clinically significant improvement in the otherwise dismal outcomes of this subset of patients. Rash is a common adverse effect associated with everolimus. However, Hand-foot syndrome is an uncommon toxicity with the use of this drug. We report a case of Grade 3 hand-foot syndrome following institution of everolimus therapy and describe its successful management.
Keywords
Breast cancer - everolimus - hand-foot syndrome - mammalian target of rapamycin inhibitors - palmar-plantar erythrodysesthesiaPublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Mammalian target of rapamycin inhibitor everolimus is a novel agent used in endocrine therapy resistant hormone receptor positive metastatic breast cancer. Its use has been associated with clinically significant improvement in the otherwise dismal outcomes of this subset of patients. Rash is a common adverse effect associated with everolimus. However, Hand-foot syndrome is an uncommon toxicity with the use of this drug. We report a case of Grade 3 hand-foot syndrome following institution of everolimus therapy and describe its successful management.
INTRODUCTION
Hormone receptor positive breast cancer has commonly been treated with selective estrogen receptor (ER) modulators, aromatase inhibitors and estrogen antagonists. However, de novo or secondary resistance to hormonal agents has for long intrigued investigators across the world. Deregulation of the phosphatidylinositol 3-kinase/protein kinase-B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway has been described to play a pivotal role in this phenomenon.[1] Everolimus, an mTOR inhibitor that acts by allosteric binding to mTORC1 protein is a recently approved therapeutic alternative for hormone therapy resistant metastatic breast cancer.[2]
CASE REPORT
A 45-year-old premenopausal female presented in October, 2007 with the complaints of persistent back pain for 5 months and was diagnosed with metastatic carcinoma right breast (ER, progesterone receptor positive, human epidermal growth factor receptor-2 (Her-2/neu negative). At presentation, she had metastases to multiple ribs and thoraco-lumbar spine. There was no evidence of visceral metastases. She received six cycles of chemotherapy with epirubicin and cyclophosphamide in association with 4-weekly intravenous zoledronic acid and daily calcium carbonate. Postchemotherapy evaluation revealed reduction in the intensity and number of her skeletal metastasis and regression of the primary breast mass, with considerable symptom relief. A simple mastectomy was performed in April, 2008 and tamoxifen was started. Seven months later (November, 2008) new bone metastasis in the mid shaft of the femur was diagnosed. Bilateral salpingo-oophorectomy was done, and an aromatase inhibitor letrozole was started. Four years and 9 months later (August, 2013) she was detected to have further progression of osseous metastases. There was no evidence of visceral metastasis. Everolimus (5 mg/day) was added to letrozole.
On the review after 1 month, she complained of recent onset itchy lesions over her both palms and soles, which progressed to skin peeling and ulceration of fingers [Figure 1] and toe tips [Figure 2]. On examination, she was found to have vesicles and crusted pustules with subungual involvement. There were no features suggestive of calcinosis/scleroderma, and no neurological or vascular deficits were found. Gram-stain of the discharge revealed many Gram-positive cocci in pairs and chains with pus cells. A diagnosis of palmar-plantar erythrodysesthesia with superadded infection was made. Everolimus was withheld, and the patient was managed with oral doxycycline 100 mg twice daily for 5 days along with topical ointment of mupirocin 2% w/w and Fluticasone 0.005% w/w on the lesions twice a day for 2 weeks. There was complete resolution of all the lesions with only residual superficial desquamation. This was further managed with topical application of 2.5% benzoyl peroxide jelly once daily and clindamycin 1% ointment twice a day for 1 week. The patient was re-challenged with everolimus and the drug was well tolerated thereafter. There was no progression of the disease at a follow-up visit at 6 months.
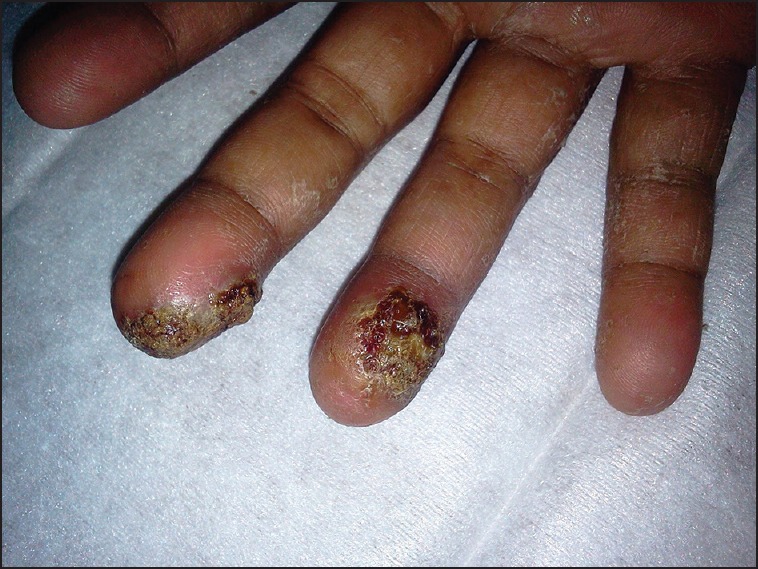
| Fig. 1 Right palm with skin peeling and crusted pustules
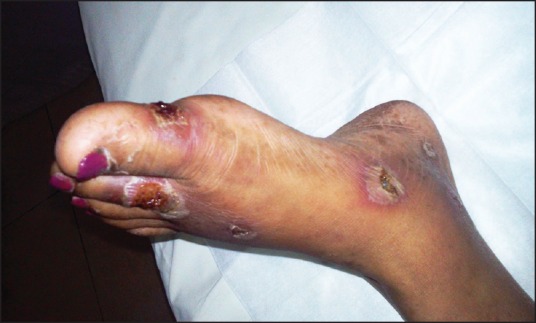
| Fig. 2 Left foot showing moist desquamation, blistering and ulceration
DISCUSSION
In hormone receptor positive breast cancer, signals communicated through ER and HER are modulated by the PI3K/Akt/mTOR pathway, which is critical in the clinical response of this group to antiendocrine therapy.[3,4] The clear clinical benefit of adding an mTOR inhibitor like everolimus has been demonstrated in multiple phase II trials.[5,6] The phase III BOLERO-2 trial showed that median progression free survival almost tripled in the everolimus arm (11.0 vs. 4.1 months; hazard ratio = 0.38 (95% confidence interval 0.31-0.48); P < 0.0001).[7] The US-Food and Drug Administration in 2012 approved everolimus in combination with aromatase inhibitor exemestane for treatment of postmenopausal women with advanced hormone-receptor positive, HER-2-neu negative breast cancer.
Palmar-plantar erythrodysesthesia, also referred to as hand-foot syndrome, encompasses a wide array of presentations which include dysesthesia, paresthesia, erythema, swelling, pain, blistering, ulceration, and desquamation in the palms of the hands or the soles of feet.[8] Grade 3 Palmar-plantar erythrodysesthesia is distinguished by severe skin changes, e.g., peeling, blisters, bleeding, edema or hyperkeratosis associated with pain and limiting self-care and activities of daily living common terminology criteria for adverse events (CTCAE), Version 4.0, June 2010, National Institutes of Health, National Cancer Institute. It has been commonly described in association with a multitude of antineoplastic agents including tyrosine kinase inhibitors, 5-fluorouracil, cytarabine, capecitabine, doxorubcin, epirubicin, interleukin-2, fluorodeoxyuridine, hydroxyurea, mercaptopurine, cyclophosphamide and docetaxel.
The most common Grade 3-4 adverse events reported among patients receiving everolimus were stomatitis, infections, hyperglycemia, rash, fatigue, diarrhea and decreased appetite. Among cutaneous toxicities, rash of any grade was reported in 36% of patients and 1% developed Grade 3 rash.[9] A meta-analysis involving a total of 2242 patients receiving everolimus for various malignancies from 13 clinical trials reported incidences of all-grade and high-grade rash at 28.6% (95% CI, 20.8-38.0) and 1.0% (95% CI, 0.6-1.8), respectively.[10] Hand-foot syndrome has been described to occur rather infrequently in patients on everolimus for metastatic renal cell carcinoma.[11]
Immediate dose reduction or cessation of the provoking agent is the most important step in management of hand-foot syndrome. Supportive measures to reduce pain and discomfort, and prevent secondary infection are very important. Given the efficacy of everolimus in hormone receptor positive metastatic breast cancer, it is crucial for physicians to recognize toxicities related to everolimus and start timely interventions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.

| Fig. 1 Right palm with skin peeling and crusted pustules

| Fig. 2 Left foot showing moist desquamation, blistering and ulceration


 PDF
PDF  Views
Views  Share
Share

