Oxaliplatin-related neuropathy in Indian patients – no difference between generic and original molecules
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 271-277
DOI: DOI: 10.4103/0971-5851.195745
Abstract
Background: Oxaliplatin-induced neuropathy is a dose-limiting toxicity that significantly affects patients' quality of life. The aim of this study was to compare its occurrence between a generic versus the original molecule in Indian patients. Materials and Methods: Between August 2012 and July 2013, 163 patients receiving oxaliplatin were prospectively enrolled. A data recording form was used in the clinic to record detailed information. Results: The median age of patients was 55 years (range, 19–79). Chemotherapy regimens used included: capecitabine, oxaliplatin (59), epirubicin, oxaliplatin, and capecitabine (20), docetaxel, oxaliplatin, and capecitabine (11), 5-FU, leucovorin, oxaliplatin (9), and gemcitabine-oxaliplatin (64). The median cumulative dose of oxaliplatin was 780 mg/m2. Eighty patients received the original version and 83 the generic one. Overall, 63 patients (38%) developed neuropathy. There was no significant difference in the incidence of neuropathy between the two forms of oxaliplatin used (P = 0.50). Forty-nine percent of female patients had neuropathy as compared to 30% of male patients (P = 0.014). Older patients had a trend toward a higher incidence of neuropathy: 44% of patients above age fifty developed neuropathy compared to 30% of patients younger than 50 (P = 0.06). Conclusion: This is the first study to specifically show that neuropathy rates do not vary with the use of generic versus original oxaliplatin.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Oxaliplatin-induced neuropathy is a dose-limiting toxicity that significantly affects patients’ quality of life. The aim of this study was to compare its occurrence between a generic versus the original molecule in Indian patients.
Materials and Methods:
Between August 2012 and July 2013, 163 patients receiving oxaliplatin were prospectively enrolled. A data recording form was used in the clinic to record detailed information.
Results:
The median age of patients was 55 years (range, 19–79). Chemotherapy regimens used included: capecitabine, oxaliplatin (59), epirubicin, oxaliplatin, and capecitabine (20), docetaxel, oxaliplatin, and capecitabine (11), 5-FU, leucovorin, oxaliplatin (9), and gemcitabine-oxaliplatin (64). The median cumulative dose of oxaliplatin was 780 mg/m2. Eighty patients received the original version and 83 the generic one. Overall, 63 patients (38%) developed neuropathy. There was no significant difference in the incidence of neuropathy between the two forms of oxaliplatin used (P = 0.50). Forty-nine percent of female patients had neuropathy as compared to 30% of male patients (P = 0.014). Older patients had a trend toward a higher incidence of neuropathy: 44% of patients above age fifty developed neuropathy compared to 30% of patients younger than 50 (P = 0.06).
Conclusion:
This is the first study to specifically show that neuropathy rates do not vary with the use of generic versus original oxaliplatin.
INTRODUCTION
Oxaliplatin is a key drug in the treatment of patients with many malignancies, including colorectal, stomach, pancreatic, and biliary tract cancers.[1,2,3,4] The drug has a unique pattern of toxicity, including acute events such as laryngeal spasm, cold-associated dysesthesia, muscle spasms, and hypersensitivity reaction, as well as chronic side effects, such as sensory neuropathy which sometimes may continue to worsen even after the drug is stopped.[5,6,7] Chronic neuropathy is the most frequent dose-limiting toxicity of oxaliplatin and can significantly affect patients’ quality of life.[5,7] Factors such as malnutrition and genetic variations can also affect the incidence of neuropathy. A higher incidence of vincristine-induced neurotoxicity has been noted in Indian children compared to the West.[8] Hertz et al. showed that there is an increased risk of paclitaxel-induced neuropathy in patients who carry the CYP2C8*3 variant in two racially distinct patient cohorts.[9]
The cost of oncology drugs is a major challenge worldwide but particularly so in resource-limited settings, hindering access to treatment. Because drug development is risky, slow, and expensive, patents are granted as an incentive to promote it, allowing recovery of the original investment during a period in which only the originator version can be sold. Once a patent expires, other companies can make and market generic drugs, as long as their product is bioequivalent. The price of medications usually drops significantly, often by 80% or more, allowing a larger number of patients to benefit from it. In the subcontinent, the approval process for generic drugs is regulated by the Drug Controller General of India. Hence, quality generic drugs will allow resource-constrained health-care systems to increase access to cancer treatments. While there are many studies and meta-analyses assessing the role of generic drugs in cardiology, little has been published in cancer care. In a retrospective study of 393 patients treated with generic docetaxel, oxaliplatin, irinotecan, and gemcitabine, the survival and toxicity were similar to the respective originator molecules.[10] A study from Qatar with colorectal cancer patients treated with oxaliplatin showed that those who received the generic version had slightly higher toxicity but the difference was not statistically significant.[11]
Moreover, worldwide, there have been shortages of chemotherapy drugs which can compromise the delivery of care for cancer patients; hence more data on the use of generic drugs are required.[12] Finally, there are currently no data on the toxicity of oxaliplatin in Indian patients. The economic background of patients in India is diverse with several generics available at affordable prices. It was important to see if toxicity between the two versions of the drug was any different. The implications in terms of morbidity and continuation of treatment would be important.
Although this is a single institution study and as such a true incidence cannot be computed, patients attending Tata Memorial Hospital are representative of the general population. It is important to compare our experience in India with published data in other parts of the world.
The aim of this study was to assess acute and chronic oxaliplatin-associated neuropathy and to compare the generic versus the original, also called reference, version of the drug in Indian patients.
MATERIALS AND METHODS
Patients
Between August 2012 and July 2013, 163 patients receiving oxaliplatin for gastrointestinal malignancies were prospectively enrolled in the study: Colorectal cancer (n = 52), biliary tract cancers (n = 59), pancreatic cancer (n = 7), stomach cancer (n = 43), and others (n = 2). Patients were chemotherapy naïve. Patients with preexisting neuropathy which was mild sensory and did not have any functional impairment were included. For all patients, oxaliplatin was discontinued if patients were to develop Grade 3 neuropathy.
All patients were treated with unified protocols [Appendix 1] within the medical oncology unit of the gastrointestinal disease management group at Tata Memorial Centre, Mumbai. Patients were reviewed in the outpatient department and received chemotherapy in the daycare area unless they were admitted overnight for desensitization protocol of oxaliplatin.
Treatment
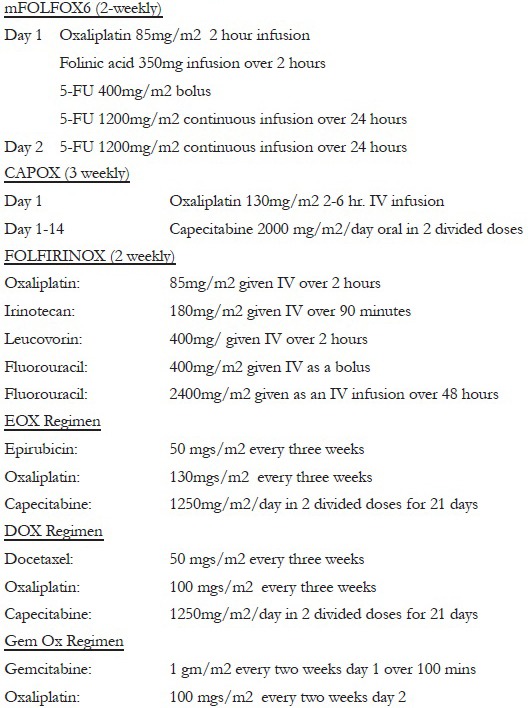
Patients could choose from the reference version of the drug (Brand name: Dacotin produced by Dr. Reddy's Laboratory) or the generic oxaliplatin (Brand name: Oxiplat produced by Sun Pharmaceuticals). There are 16 different generic brands of oxaliplatin manufactured in India and 31 listed in the Medindia database (http://www.medindia.net/drug-price/oxaliplatin.htm). The choice of generic was based on what was available in the institutional pharmacy. Dacotin costs approximately 7–8 times more than oxiplat, a very important factor in deciding which drug will be administered as the majority of treatment in India is self-funded. Patients who received dacotin were either economically rich or had third-party insurance which funded the treatment. The diagnosis, chemotherapy regimen used, frequency of regime, and dose of oxaliplatin administered in each patient were recorded. The chemotherapy regimens used were gemcitabine-oxaliplatin (Gem-Ox) at fortnightly intervals for biliary tract cancers, epirubicin, oxaliplatin, and capecitabine (EOX) at 3 weekly intervals for stomach cancer, docetaxel, oxaliplatin, and capecitabine (DOX) at fortnightly intervals for stomach cancer, 5-FU, leucovorin, oxaliplatin (mFOLFOX6) at fortnightly intervals for colorectal cancers, capecitabine, oxaliplatin (Cape-Ox) at 3 weekly intervals for stomach and colorectal cancers, and 5-FU, oxaliplatin, irinotecan, leucovorin at fortnightly intervals for pancreatic cancers. Cumulative doses of oxaliplatin per patient were calculated.
Ethics
The data of the present study were collected in the course of common clinical practice, and accordingly, the signed informed consent was obtained from each patient for the chemotherapy administered. The Ethics Committee approval was not taken for the study as this was an audit carried out within the department.
Dose modifications
When an infusion-related hypersensitivity reaction occurred, the infusion was interrupted and supportive care given in the form of corticosteroids, antihistamines, and epinephrine as indicated. If the reaction was severe, patients were admitted for subsequent cycles for an oxaliplatin desensitization protocol [Appendix 2].[6] If patients developed Grade 2 toxicity, the dose of oxaliplatin was reduced in subsequent cycles, but if the patient developed Grade 3 toxicity which limited activities of self-care daily living, further infusions were discontinued. Doses were not reduced for reversible Grade 1 neuropathy.
Neuropathy assessment
Patients were assessed for grades of neuropathy according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.[13] Assessment was done at baseline and during chemotherapy after the first cycle and then alternate cycles. Grade 1 neuropathy was defined as mild paresthesia, loss of deep tendon reflexes, Grade 2 moderate paresthesia, mild or moderate objective sensory loss, and Grade 3 neuropathy-paresthesia interfering with function, severe objective sensory loss. Grade 4 neuropathy was defined as debilitating permanent sensory and motor disability. The data recording form used for the assessment of neurotoxicity is shown in Appendix 3.
Statistics
Results are presented in the form of counts and percentages. The χ2 test was used to compare proportions in different categories. Nonparametric test was used to compare medians. Formal sample size calculation was not done.
RESULTS
Patient characteristics
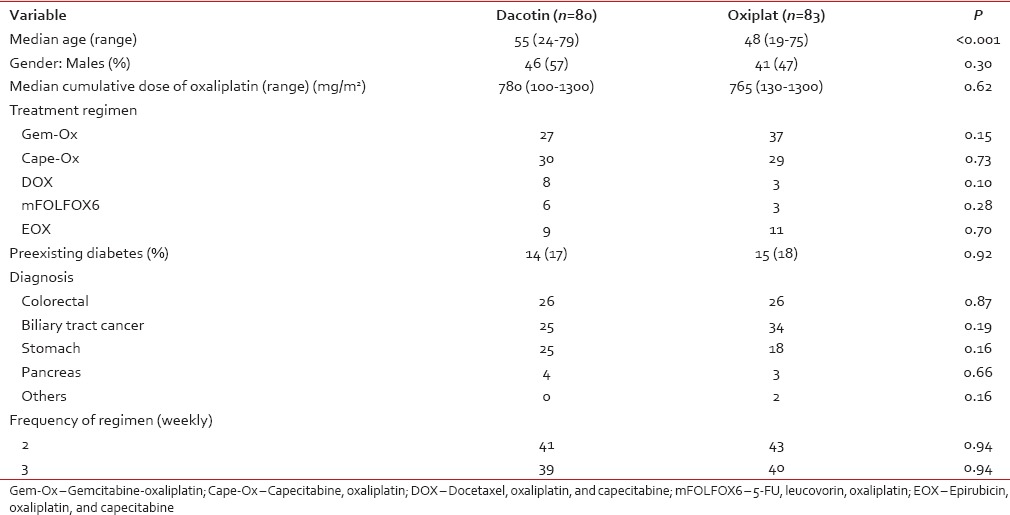
The median age of patients who received dacotin was 55 years versus 48 years for the group that received oxiplat (range, 19–79 years; P < 0.001). Eighty-seven of 163 patients were (53%) men. Table 1 shows the demographic and baseline characteristics of all patients included. Twenty-nine patients had diabetes at diagnosis with no statistical difference between the patients who received either version of oxaliplatin (P = 0.92).
Table 1
Patient demography
Treatment
The median cumulative dose of oxaliplatin was 780 mg/m2 (range, 100–1300 mg/m2). There was no difference in the cumulative dose received between groups (P = 0.62) as shown in Table 1. Fifty-nine patients received Cape-Ox, 64 received Gem-Ox, 11 patients received DOX, 20 received EOX, and 9 received mFOLFOX6.
Neuropathy
Risk factors
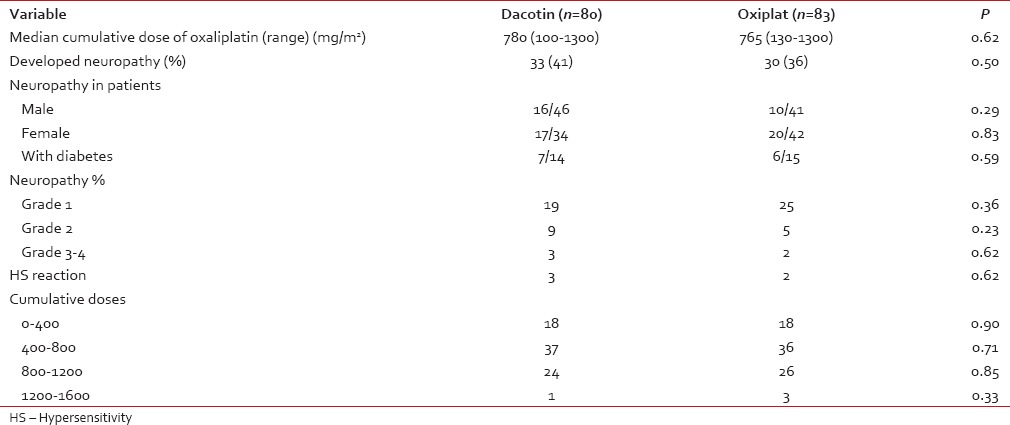
Of 163 patients, 63 (38%) developed Grade 1–3 neuropathy. Forty-six individuals developed neuropathy, while on chemotherapy, 13 developed neuropathy postchemotherapy and 4 had worsening of preexisting neuropathy. Of 76 female patients, 37 (49%) developed neuropathy compared to 26 of 87 male patients (30%; P = 0.014). 44 of 99 (44%) patients >50 years developed neuropathy compared to 19 of 64 (30%) patients < 50 years of age (P = 0.06).
The incidence of peripheral neuropathy did not differ markedly in the regimens used – Cape-Ox: 21/59 (35%), DOX: 5/11 (45%), EOX: 6/20 (30%), mFOLFOX6: 4/9 (44%), Gem-Ox: 27/64 (42%).
Table 2
Comparison of neuropathy between dacotin and oxiplat
Dose modifications
Grade 1 neuropathy was seen in 44 (70%) patients, Grade 2 in 14 (22%), and Grade 3 in 5 (8%) patients. Of the five patients with Grade 3 neuropathy, three developed this at the end of therapy, hence stopped therapy anyway while two patients were discontinued further oxaliplatin. Sixteen (25%) patients required dose reduction, 14 of 16 patients required dose reduction for Grade 2 neuropathy while 2/16 patients with preexistent neuropathy were given dose-reduced treatment. Severe allergic reactions were seen in five patients during oxaliplatin infusion. The desensitization protocol [Appendix 2] was used in all of them successfully.
Reference versus generic drug
The original molecule, dacotin, was administered to 80 patients, while 83 individuals received oxiplat, the generic. As shown in Table 2, although the patients in the dacotin group were significantly younger, there was no difference in the incidence of neuropathy between the two brands of drug used.
DISCUSSION
Oxaliplatin is a commonly used drug in gastrointestinal malignancies. Worldwide, oxaliplatin neuropathy has been addressed extensively,[14] but there is limited information of its use in an Indian setting. Oxaliplatin neuropathy was seen in 38% of patients in our study which is comparable to the range between 24% and 49% of patients noted in Western studies.[14,15]
In our study, female patients had a significantly increased incidence of neuropathy. We also observed a trend for the higher incidence of neuropathy in individuals older than 50 years of age. Alejandro et al. analyzed patients receiving mFOLFOX and on univariate analysis, they found female sex, younger age, and body surface area >2.0 were potentially associated with acute neuropathy though this did not hold true on multivariate analysis; and greater body mass index and persistent neuropathy in the past cycle were potentially associated with persistent neuropathy.[16] This study also reported that if adjusted for the winter season and creatinine clearance, older patients were less likely to report neuropathy. A pooled analysis of individual patient data for patients with colorectal cancer treated with FOLFOX4 showed that age was not a risk factor for neuropathy.[17]
Some studies have suggested that the presence of diabetes could lower the threshold of developing neuropathy but does not usually affect its severity.[18] In our study, the presence of diabetes had no effect on the incidence or severity of neuropathy. The fortnightly versus 3 weekly administration of oxaliplatin, type of cancer, and cumulative doses of oxaliplatin, also did not have any impact on the incidence of the neuropathy. The severity of neuropathy increased with an increase in cumulative doses as has been shown before.
This study has its own limitations, chief of which was a relatively small sample size. We need further prospective randomized studies to consolidate these results. We also did not assess the outcome and response of patients who required dose reductions due to neuropathy.
CONCLUSION
We showed that female patients have increased risk of developing neuropathy, as may do those older than 50 years of age. More importantly, there were no differences in neuropathy in the comparison between the reference and the generic drugs. Patients can safely use the generic version of oxaliplatin but there are numerous generic versions available and which one to use can depend on certain criteria – whether the pharmaceutical company has a WHO GMP accredited facility and if the drug has been approved by the National Drug Controller Authority and if there are some research and development portfolio of the pharmaceutical company.
Financial support and sponsorship
Nil.
Conflicts of interest
Appendix 1: Description of regimens used
Appendix 2: Oxaliplatin desensitisation

Appendix 3: Questionnaire



 PDF
PDF  Views
Views  Share
Share

