Oxaliplatin induced Peripheral Neuropathy in South Indian Cancer Patients: A Prospective Study in Digestive Tract Cancer Patients
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 502-507
DOI: DOI: 10.4103/ijmpo.ijmpo_143_17
Abstract
Purpose: The aim of the current study is to report our prospective experience on the prevalence of oxaliplatin-induced peripheral neuropathy (OXAIPN) in patients with digestive tract cancers treated with oxaliplatin-basedcombination therapy. Materials and Methods: A total of 219 patients scheduled to be treated with oxaliplatin-basedcombination therapy were prospectively examined at baseline and follow-up during the therapy between November 2014 and December 2016. The incidence of acute OXAIPN was measured using a descriptive questionnaire (yes/no question) based on sum of number of symptoms present and NCI-CTCAE version 4.03 was applied to clinically grade the severity of chronic OXAIPN. Results: Acute and chronic OXAIPN was found in 108 of 219 (49.3%) and 127 of 219 (58%) patients, respectively. Out of 11 acute OXAIPN symptoms, the vast majority of patients manifested cold-induced pharyngolaryngeal (63.8%) dysesthesias or perioral (61.1%) paresthesias. Development of acute OXAIPN was predictive of subsequent development of chronic OXAIPN (P = 0.0001). All the patients received a median cumulative dose of 780 mg/m2 (range: 130–1040 mg/m2). There was a significant correlation between the patients who received the median cumulative dose and the development of chronic OXAIPN. The incidences of OXAIPN in patients with median cumulative dose of ≤780 mg/m2 was 51/120 (42.5%) and >780 mg/m2 was OXAIPN 76/99 (76.7%) (P = 0.0001). Conclusion: The current study results demonstrate that the vast majority of patients who receive oxaliplatin-basedcombination chemotherapy will manifest acute OXAIPN that may contribute to the development of chronic peripheral neuropathy on repeated courses of drug administration.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Purpose:
The aim of the current study is to report our prospective experience on the prevalence of oxaliplatin-induced peripheral neuropathy (OXAIPN) in patients with digestive tract cancers treated with oxaliplatin-based combination therapy.
Materials and Methods:
A total of 219 patients scheduled to be treated with oxaliplatin-based combination therapy were prospectively examined at baseline and follow-up during the therapy between November 2014 and December 2016. The incidence of acute OXAIPN was measured using a descriptive questionnaire (yes/no question) based on sum of number of symptoms present and NCI-CTCAE version 4.03 was applied to clinically grade the severity of chronic OXAIPN.
Results:
Acute and chronic OXAIPN was found in 108 of 219 (49.3%) and 127 of 219 (58%) patients, respectively. Out of 11 acute OXAIPN symptoms, the vast majority of patients manifested cold-induced pharyngolaryngeal (63.8%) dysesthesias or perioral (61.1%) paresthesias. Development of acute OXAIPN was predictive of subsequent development of chronic OXAIPN (P = 0.0001). All the patients received a median cumulative dose of 780 mg/m2 (range: 130–1040 mg/m2). There was a significant correlation between the patients who received the median cumulative dose and the development of chronic OXAIPN. The incidences of OXAIPN in patients with median cumulative dose of ≤780 mg/m2 was 51/120 (42.5%) and >780 mg/m2 was OXAIPN 76/99 (76.7%) (P = 0.0001).
Conclusion:
The current study results demonstrate that the vast majority of patients who receive oxaliplatin-based combination chemotherapy will manifest acute OXAIPN that may contribute to the development of chronic peripheral neuropathy on repeated courses of drug administration.
Introduction
Oxaliplatin, a newer platinum anticancer drug that differs both structurally and in the spectrum of activity from other platinum drugs, has been in use in the treatment of various digestive tract cancers.[1] It is often used along with other drugs as a combination regimen. Oxaliplatin-based combination regimens such as FOLFOX, CAPOX, and EOX which consists of leucovorin, 5-fluorouracil and oxaliplatin; capecitabine, oxaliplatin and epirubicin, capecitabine and oxaliplatin; respectively have demonstrated prolonged progression-free survival and overall survival in both early as well as advanced disease of the gastrointestinal tract.[2,3,4,5,6,7]
One of the main dose-limiting toxicities for oxaliplatin is peripheral neuropathy (PN) which affects nearly 75% of all treated patients.[8] Oxaliplatin-induced PN (OXAIPN) can be characterized into two distinct types. One is an acute type which is transient and predominantly sensory and may be aggravated or precipitated by cold exposure. It has a rapid onset and lasts from few hours to days following treatment but is reversible. The second type is the chronic PN. It is sensory in nature, gradually progressive, and incidence increase with increasing the cumulative dose of oxaliplatin at around 700 mg/m2. It is characterized by sensory paresthesias and dysesthesias which may impair daily activities of the patient.[9,10,11,12,13,14]
The development of acute form is believed to be due to dysfunction of nodal axonal voltage-gated sodium channels and probably the calcium channels. The rapid chelation of calcium by oxaliplatin-induced oxalate and the subsequent alteration in voltage-dependent sodium channel kinetics could be the main reason that generates the acute OXAIPN. Conversely, oxalaiplatin accumulation in sensory neurons of dorsal root ganglia (DRG) may result in chronic OXAIPN by damaging the DRG with axoplasmatic transport changes. In addition, oxidative stress from mitochondrial dysfunction also may contribute significantly to OXAIPN through neuronal apoptosis.[15,16]
To date, there have been very few studies in literature which attempted to define predictive markers for OXAIPN and efforts to identify effective prophylactic or therapeutic agents but were inconclusive. Identification of an accurate marker that would provide or predict the incidence or severity of OXAIPN can be useful in controlling this toxicity. This study was aimed to report on the incidence and severity of both the acute and chronic OXAIPN in South Indian cancer patients treated with oxaliplatin-based combination regimen to investigate the association of OXAIPN with baseline as well as clinical characteristics.
Materials and Methods
Study design
It is a prospective, single institutional study conducted in the Departments of Medical Oncology, Pharmacology, and Neurology at Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. The patients were recruited during a 26-month period from out patient's Department of Medical Oncology, between November 2014 and December 2016.
Patient selection
Patients with histologically confirmed gastric or colorectal cancer, scheduled to receive oxaliplatin-based combination (Modified FOLFOX-4, CAPOX, and EOX) either as adjuvant, neoadjuvant treatment or as palliative treatment were prospectively screened. The following were the inclusion criteria: Patients who have not received oxaliplatin previously, normal liver (bilirubin: 0.3–1.9 mg/dL; alanine transaminase and aspartate transaminase: 7–40 U/L and 10–40 U/L) and renal functions serum creatinine: 0.6–1.1 mg/dL in women and 0.7–1.3 mg/dL in men, blood urea nitrogen: 7–20 mg/dL), normal electrophysiological profile (Nerve conduction velocity, amplitude, distal latency) of unilateral (Right side) sural, common peroneal and ulnar nerves. The exclusion criteria were patients aged <18>2, patient receiving other drugs causing PN.
Chemotherapy treatment and dose modification
The allocation to treatment of oxaliplatin-based regimen was made according to treating medical oncologist's discretion. For the FOLFOX regimen, oxaliplatin was given at 85 mg/m2 every 2 weeks for 12 cycles and with CAPOX and EOX regimen, patients received oxaliplatin at 130 mg/m2, every 3 weeks, for 8 cycles, respectively. Oxaliplatin was infused intravenously for 2 h for all the patients. Dose adjustments of the study drugs or treatment delays were calculated based on the development of toxicity grade. For persistent or temporary (at least 14 days) painful paresthesia, dysesthesia or functional impairment, 25% of oxaliplatin dose was reduced. If in spite of the 25% oxaliplatin dose reduction the Grade 3 or 4 neurotoxicity persisted, chemotherapy was not given in subsequent cycles.[17] Symptomatic treatment was given for neurotoxicity during the administration of chemotherapy.
Outcome measures
For the assessment of acute OXAIPN, we used a simple descriptive questionnaire (a yes/no response format) to quantify the frequency of the 11 most common hyperexcitability and transient symptoms, as previously described by Velasco et al.: 2014, and Argyriou et al.; 2013.[18,19] For the current study, severity was graded based on the sum of number of symptoms If the patients manifested 1–3 symptoms, the severity of OXAIPN was graded 1, if the symptoms were present between 4 and 6 then the severity was Grade 2 and so if 6–9 symptoms were present then it was graded 3 and more than 9 symptoms was graded as 4. Oxaliplatin-induced chronic PN (chronic OXAIPN) was graded using the National Cancer Institute common toxicity criteria, version 4.03. (NCI-CTCAE Version 4.03, America). Both the acute and chronic OXAIPN were evaluated at every cycle of treatment.
Electrophysiological profile (nerve conduction studies)
Nerve conduction studies (NCS) were done unilaterally, especially right side using standardized technique (Nihon kohden, model: MEB9200K, Tokyo, Japan). Sensory and motor conductions were evaluated in ulnar, sural and common peroneal nerves by measuring peak to peak amplitude, nerve action potentials, and conduction velocities.
Statistical analysis
Descriptive statistics were used for all variables. Comparison of categorical data among patients treated with modified FOLFOX, CAPOX, EOX, and association of baseline characteristics with incidence of OXAIPN was done using the two-sided Chi-square test. The correlation between the incidence and severity of chronic OXAIPN development and severity of acute OXAIPN was also done with Chi-square test. Oxaliplatin dose association with development of OXAIPN was done using Student's t-test. P < 0>
Results
Between November 2014 and December 2016, a total of 273 patients scheduled to receive oxaliplatin-based chemotherapy were initially screened and 219 patients eventually included in the study. During screening, 54 patients were excluded for various reasons. 39 patients were excluded due to presence of either clinical neuropathy or due to abnormal NCS results and 9 patients for receiving oxaliplatin previously and 6 patients for other reasons such as abnormal liver and kidney functions.
Among 219 patients, 137 (62.5%) were male, and 82 (37.5%) were female with a median age of 53 years (range, 19–75 years). The mean oxaliplatin dose administered to the patients was 778.5 ± 266.5 mg/m2. Detailed baseline demographics and clinical characteristics of the patients are listed in Table 1.
Table 1
Baseline demographics and clinical characteristics (n=219)
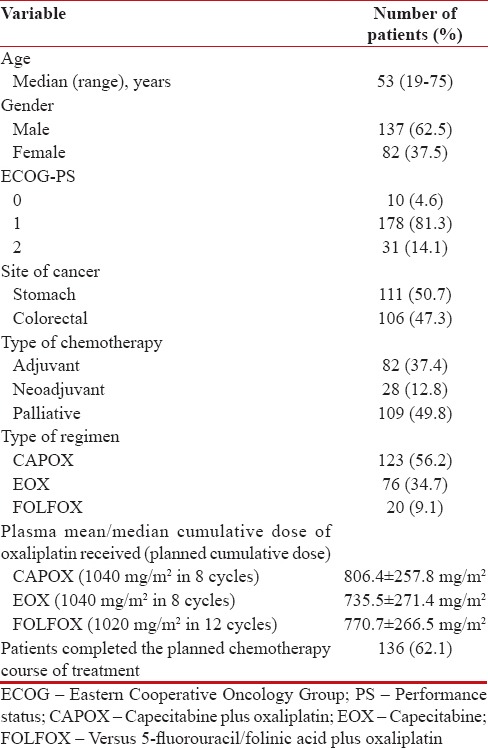
|
A total of 136 (62.1%) patients had completed planned chemotherapy treatment. There were 10 (4.6%) deaths while 48 (21.9%) patients were lost to follow-up. Treatment change was there in 25 (11.4%) patients due to disease progression (18 patients), PN (2 patients), hematological (3 patients), and gastrointestinal toxicity (2 patients).
Incidence and severity of acute oxaliplatin-induced peripheral neuropathy
Acute OXAIPN symptoms, defined as those occurring immediately or later (within 14 days) following oxaliplatin infusion, occurred in 108 of 219 (49.3%) patients. Out of 108, overall the vast majority of patients manifested cold-induced pharyngolaryngeal (63.8%) dysesthesias or perioral (61.1%) paresthesia. Development of acute OXAIPN was seen in the majority of the patients who received between cycles 0 and 6 of FOLFOX, 0 and 4 cycles of CAPOX, EOX. Table 2 summarizes the proportion along with median duration of symptoms for 11 acute OXAIPN symptoms.
Table 2
The incidence of acute oxaliplatin-induced peripheral neuropathy symptoms in the patients receiving oxaliplatin (n=108 of 219 patients; 49.3%)
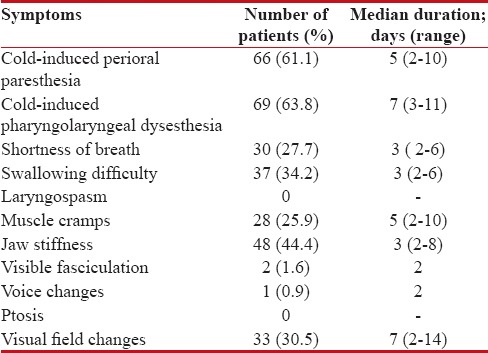
|
The average number of symptoms that patients with acute OXAIPN reported was 3 symptoms (range, 1–9 symptoms). Most common symptoms seen at first cycle were cold-induced perioral paresthesia (23 patients) and pharyngolaryngeal dysesthesia (19 patients) followed by jaw stiffness (15 patients), and difficulty in swallowing (13 patients). The maximum grade of acute OXAIPN was Grade1 in 70 (64.8%) patients, Grade 2 in 33 (30.5%) patients, and Grade 3 in 5 (4.6%) patients. The median time for onset of acute OXAIPN was 21 (1–84) days.
Incidence and severity of chronic oxaliplatin-induced peripheral neuropathy
At the end of treatment, chronic OXAIPN was present in 127 of 219 patients (58.0%). The median cumulative dose of oxaliplatin was 780 mg/m2 (range: 130–1040 mg/m2). The incidence of chronic OXAIPN in patients with median cumulative dose of ≤780 mg/m2 was 51/120 (42.5%) and >780 mg/m2 was OXAIPN 76/99 (76.7%) (P = 0.0001). The incidence of chronic OXAIPN for different regimes was 77/123 (62.6%), 42/76 (55.2%), 8/20 (40.0%) for CAPOX, EOX, and FOLFOX, respectively. The maximum grade of chronic OXAIPN was Grade 1 in 71 (55.9%) patients, Grade 2 in 54 (42.5%) patients, Grade 3 in 2 patients (1.5%). The median time to the onset of chronic OXAIPN was 105 (42–168) days.
Association of acute and chronic oxaliplatin-induced peripheral neuropathy
In total, 108 of 219 patients (49.3%) who developed acute OXAIPN, 84 (77.7%) patients eventually manifested various degrees of chronic OXAIPN while 24 (22.3%) patients did not develop any degrees of chronic OXAIPN. Of 111 patients, 43 (38.7%) patients who did not develop any degree of acute OXAIPN developed chronic OXAIPN. The incidence of acute OXAIPN was predictive of subsequent development of chronic OXAIPN (P = 0.0001) [Table 3]. However, acute OXAIPN was significantly higher in incidence in patients <53 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759072/table/T4/" target="table" class="fig-table-link figpopup" rid-figpopup="T4" rid-ob="ob-T4" co-legend-rid="" xss=removed>Table 4.
Table 3
Association of acute oxaliplatin-induced peripheral neuropathy with chronic oxaliplatin-induced peripheral neuropathy

|
Table 4
Association of acute OXAIPN and chronic OXAIPN with baseline variables
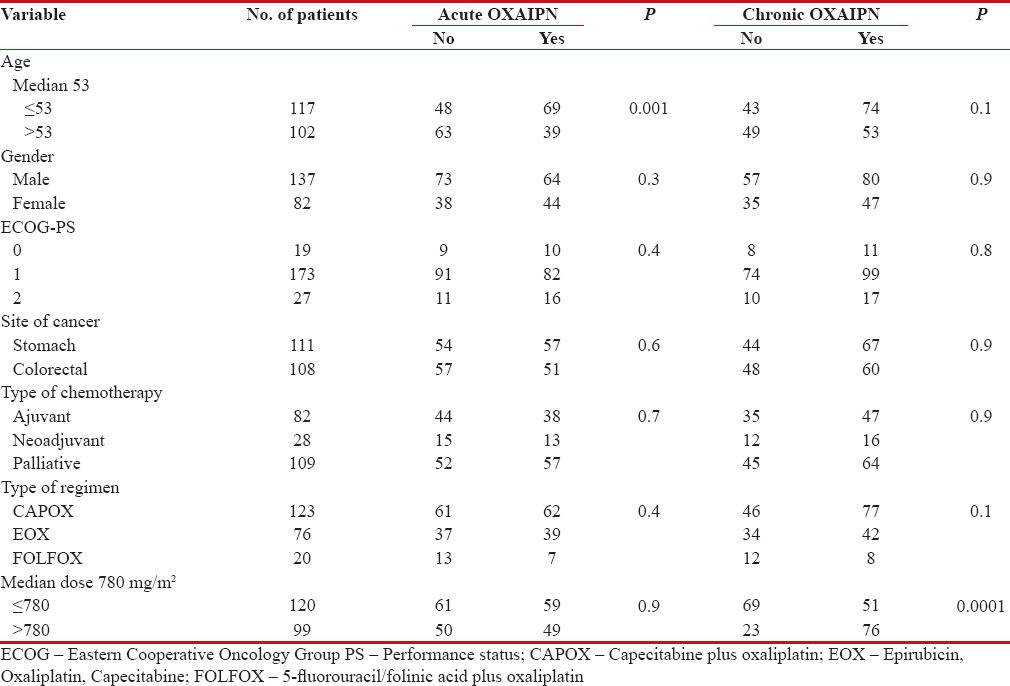
|
The incidence of chronic OXAIPN was related to the cumulative oxaliplatin dose and patients receiving >780 mg/m2 of cumulative dose of oxaliplatin had higher incidence of chronic OXAIPN. The incidence of chronic OXAIPN was not associated with other baseline and clinical factors such as age, gender, performance status, and comorbidity status, site of cancer or type of combination chemotherapy [Table 4].
Dose modification or drug infusion prolongation:
A total of 12 (11.1%) of 108 patients who developed acute OXAIPN required prolongation of oxaliplatin infusion from 2 h to 4–6 h due to development of severe acute OXAIPN. Prolongation of oxaliplatin infusion occurred at, 1st course in 4 patients, 2nd course 6 patients, 3rd course 1 patient, and 6th course 1 patient. There was 25% dose reduction in 5 (4.6%) of 108 patients after 5 courses of chemotherapy due to toxicity development. A total of 3 patients were due to hematological toxicity, and 2 patients were due to chronic PN. No patient was discontinued on planned chemotherapy protocol due to OXAIPN.
Discussion
Oxaliplatin-based combination chemotherapy is presently considered as first-line treatment in various gastrointestinal tract cancers, especially in stomach and colorectal cancers. Among nonhematological toxicities of oxaliplatin, PN is one of the main dose-limiting toxicities. Worldwide, OXAIPN has been addressed extensively.[20]
In a prospective study done by Argyriou et al., 2013 in 170 colorectal patients who were treated either with FOLFOX or CAPOX, acute OXAIPN was found in 85.9%, and chronic OXAIPN was seen in 72.4% of the patients.[19] In a retrospective study, where a total of 188 colorectal cancer patients were treated with CAPOX regimen, acute OXAIPN was seen in 94% while the chronic was seen in 57% of the patients.[21] In another recent study conducted in Iran, chronic OXAIPN was seen in 80.7% of 130 colorectal cancer patients who had received either FOLFOX or CAPOX regimens.[22] All the published studies in literature attempted to compare the neurotoxicity potential of CAPOX regimen with FOLFOX regimen and vice versa in colorectal cancer patients, and there is limited data till date regarding the incidence of OXAIPN in gastric cancer patients who are treated with EOX regimen.
In the present study, we have evaluated the overall incidence of both acute and chronic OXAIPN in digestive tract cancer patients, i.e., both stomach and colorectal cancer patients who were treated with oxaliplatin-based combination regimens such as FOLFOX, CAPOX, or EOX. We found the overall toxicity of acute OXAIPN in 49.3% and chronic OXAIPN in 58.0% of the patients. Coming to the severity of both acute OXAIPN and chronic OXAIPN, Patients with Grade 1 were more in our study when compared to Grade 2–3.(Grade 1 of acute OXAIPN was present in 64.8%, and chronic OXAIPN was seen in 55.9% of the patients). However, there is difference in the incidence and severity of both the acute and chronic OXAIPN of the present study results with other study results from West.[18,19,22,23,24,25,26] The reason behind this may be possibly due to the different genetic makeup of patient populations involved in these studies.
Studies in literature acknowledged that the degree of chronic OXAIPN is dependent on the cumulative dose and duration of drug administration.[22,26] In our study, oxaliplatin dose was significantly associated with the development of chronic OXAIPN. However, previous studies reported only the association between cumulative dose of oxaliplatin and development of chronic OXAIPN during the treatment period, but our current study results demonstrate the relationship between the cumulative dose and chronic OXAIPN as well as in between acute OXAIPN and chronic OXAIPN from cycle 1 to end of the treatment. The key findings of our study include acute OXAIPN symptoms which are developed during the treatment are reversible and may last for a maximum of 14 days; acute OXAIPN symptoms which appear on cycle one of treatment will be severe on subsequent cycles with oxaliplatin-based combination therapy; patients who experience acute OXAIPN are more prone to develop chronic OXAIPN; patients with age >53 years are less likely to report about the incidence of acute OXAIPN; chronic OXAIPN is more in patients with cumulative dose >780 mg/m2.
To date, the chronic OXAIPN was considered to have more clinical importance than acute OXAIPN as it is often dose limiting and long-lasting toxicity.[24,27] Therefore, the research has been focused on chronic OXAIPN than that of acute OXAIPN which usually is reversible and only requires the prolongation of oxaliplatin administration without requiring dose modification or discontinuation of treatment.[25] Hence, we aimed to analyze both the acute and chronic OXAIPN frequency and their features in a large, homogenous population.
Few studies have reported on the persistence of chronic OXAIPN for more than 12 months following completion of treatment.[28,29] However, in our study, we recorded the incidence of chronic OXAIPN till end of the treatment. We could not follow-up the patients after completion of the treatment. This is one of our limitations in the study. We need further prospective randomized studies to validate the current study results.
To the best of our knowledge, the current study is the first study which provides the incidence rate of both the acute and chronic OXAIPN and their association with the clinical and baseline factors in south Indian (Dravidian) patients with both gastric and colorectal cancers.
The current study results demonstrate that the vast majority of patients with digestive tract cancer who receive oxaliplatin-based combination chemotherapy will manifest a transient, acute hyperexcitability syndrome (49.3%) that may contribute to the development of chronic PN (58.0%) due to repeated courses of drug administration. Hence the clinicians should pay special attention toward the incidence of PN in digestive tract cancers patients who receive oxaliplatin-based regimen. Pharmacogenetics profiling study in these patients is underway to stratify the patients who develop OXAIPN for the administration of oxaliplatin at the right dose and the right time.
Ethical approval and Informed consent
This study was approved by local Institutional Ethical Committee (Reg. No: ECR/342/Inst/PY/2013), JIPMER and JIPMER Scientific Advisory Committee (No. JSAC 15/06/2014). The study was performed in accordance with the ethical standards of the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hill EJ, Nicolay NH, Middleton MR, Sharma RA. Oxaliplatin as a radiosensitiser for upper and lower gastrointestinal tract malignancies: What have we learned from a decade of translational research? Crit Rev Oncol Hematol 2012;83:353-87.
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51.
- Pilancı KN, Saglam S, Okyar A, Yucel S, Pala-Kara Z, Ordu C, et al. Chronomodulated oxaliplatin plus capecitabine (XELOX) as a first line chemotherapy in metastatic colorectal cancer: A Phase II brunch regimen study. Cancer Chemother Pharmacol 2016;78:143-50.
- Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21.
- Chao Y, Hsieh JS, Yeh HT, Su YC, Wu CC, Chen JS, et al. Amulticenter phase II study of biweekly capecitabine incombination with oxaliplatin as first-line chemotherapy in patients with locally advanced or metastatic gastric cancer. Cancer Chemother Pharmacol 2014;73:799-806.
- Wang Y, Zhuang RY, Yu YY, Yu S, Hou J, Ji Y, et al. Efficacy of preoperative chemotherapy regimens in patients with initially unresectable locally advanced gastric adenocarcinoma: Capecitabine and oxaliplatin (XELOX) or with epirubicin (EOX). Oncotarget 2016;7:76298-307.
- Wang ZX, Yang XL, He MM, Wang F, Zhang DS, Li YH, et al. The efficacy of adjuvant FOLFOX6 for patients with gastric cancer after D2 lymphadenectomy: A Propensity score-matched analysis. Medicine (Baltimore) 2016;95:e3214.
- Argyriou AA. Updates on oxaliplatin-induced peripheral neurotoxicity (OXAIPN). Toxics 2015;3:187-97.
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006;33:15-49.
- Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve 2004;29:387-92.
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 2009;8:10-6.
- ;Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin 2013;63:419-37.
- Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999-2007.
- Petrioli R, Pascucci A, Francini E, Marsili S, Sciandivasci A, Tassi R, et al. Neurotoxicity of FOLFOX-4 as adjuvant treatment for patients with colon and gastric cancer: A randomized study of two different schedules of oxaliplatin. Cancer Chemother Pharmacol 2008;61:105-11.
- Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, et al. Platinum-induced neurotoxicity and preventive strategies: Past, present, and future. Oncologist 2015;20:411-32.
- Canta A, Pozzi E, Carozzi VA. Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 2015;3:198-223.
- Leonard GD, Wright MA, Quinn MG, Fioravanti S, Harold N, Schuler B, et al. Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC Cancer 2005;5:116.
- Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P, et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 2014;85:392-8.
- Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: A prospective study in 170 patients with colorectal cancer. Cancer 2013;119:438-44.
- Grothey A. Oxaliplatin-safety profile: Neurotoxicity. Semin Oncol 2003;30:5-13.
- ;Storey DJ, Sakala M, McLean CM, Phillips HA, Dawson LK, Wall LR, et al. Capecitabinecombined with oxaliplatin (CapOx) in clinical practice: How significant is peripheral neuropathy? Ann Oncol 2010;21:1657-61.
- Shahriari-Ahmadi A, Fahimi A, Payandeh M, Sadeghi M. Prevalence of oxaliplatin-induced chronic neuropathy and influencing factors in patients with colorectal cancer in iran. Asian Pac J Cancer Prev 2015;16:7603-6.
- Burakgazi AZ, Messersmith W, Vaidya D, Hauer P, Hoke A, Polydefkis M, et al. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology 2011;77:980-6.
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer 2008;44:1507-15.
- Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, et al. Clinical course of oxaliplatin-induced neuropathy: Results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 2015;33:3416-22.
- Argyriou AA, Velasco R, Briani C, Cavaletti G, Bruna J, Alberti P, et al. Peripheral neurotoxicity of oxaliplatin incombination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): A prospective evaluation of 150 colorectal cancer patients. Ann Oncol 2012;23:3116-22.
- Baek KK, Lee J, Park SH, Park JO, Park YS, Lim HY, et al. Oxaliplatin-induced chronic peripheral neurotoxicity: A prospective analysis in patients with colorectal cancer. Cancer Res Treat 2010;42:185-90.
- Pietrangeli A, Leandri M, Terzoli E, Jandolo B, Garufi C. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol 2006;56:13-6.
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC, et al. Long-term neuropathy after oxaliplatin treatment: Challenging the dictum of reversibility. Oncologist 2011;16:708-16.
References
- Hill EJ, Nicolay NH, Middleton MR, Sharma RA. Oxaliplatin as a radiosensitiser for upper and lower gastrointestinal tract malignancies: What have we learned from a decade of translational research? Crit Rev Oncol Hematol 2012;83:353-87.
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51.
- Pilancı KN, Saglam S, Okyar A, Yucel S, Pala-Kara Z, Ordu C, et al. Chronomodulated oxaliplatin plus capecitabine (XELOX) as a first line chemotherapy in metastatic colorectal cancer: A Phase II brunch regimen study. Cancer Chemother Pharmacol 2016;78:143-50.
- Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21.
- Chao Y, Hsieh JS, Yeh HT, Su YC, Wu CC, Chen JS, et al. Amulticenter phase II study of biweekly capecitabine incombination with oxaliplatin as first-line chemotherapy in patients with locally advanced or metastatic gastric cancer. Cancer Chemother Pharmacol 2014;73:799-806.
- Wang Y, Zhuang RY, Yu YY, Yu S, Hou J, Ji Y, et al. Efficacy of preoperative chemotherapy regimens in patients with initially unresectable locally advanced gastric adenocarcinoma: Capecitabine and oxaliplatin (XELOX) or with epirubicin (EOX). Oncotarget 2016;7:76298-307.
- Wang ZX, Yang XL, He MM, Wang F, Zhang DS, Li YH, et al. The efficacy of adjuvant FOLFOX6 for patients with gastric cancer after D2 lymphadenectomy: A Propensity score-matched analysis. Medicine (Baltimore) 2016;95:e3214.
- Argyriou AA. Updates on oxaliplatin-induced peripheral neurotoxicity (OXAIPN). Toxics 2015;3:187-97.
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 2006;33:15-49.
- Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve 2004;29:387-92.
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 2009;8:10-6.
- ;Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J Clin 2013;63:419-37.
- Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 2014;22:1999-2007.
- Petrioli R, Pascucci A, Francini E, Marsili S, Sciandivasci A, Tassi R, et al. Neurotoxicity of FOLFOX-4 as adjuvant treatment for patients with colon and gastric cancer: A randomized study of two different schedules of oxaliplatin. Cancer Chemother Pharmacol 2008;61:105-11.
- Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, et al. Platinum-induced neurotoxicity and preventive strategies: Past, present, and future. Oncologist 2015;20:411-32.
- Canta A, Pozzi E, Carozzi VA. Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 2015;3:198-223.
- Leonard GD, Wright MA, Quinn MG, Fioravanti S, Harold N, Schuler B, et al. Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC Cancer 2005;5:116.
- Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P, et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 2014;85:392-8.
- Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: A prospective study in 170 patients with colorectal cancer. Cancer 2013;119:438-44.
- Grothey A. Oxaliplatin-safety profile: Neurotoxicity. Semin Oncol 2003;30:5-13.
- ;Storey DJ, Sakala M, McLean CM, Phillips HA, Dawson LK, Wall LR, et al. Capecitabinecombined with oxaliplatin (CapOx) in clinical practice: How significant is peripheral neuropathy? Ann Oncol 2010;21:1657-61.
- Shahriari-Ahmadi A, Fahimi A, Payandeh M, Sadeghi M. Prevalence of oxaliplatin-induced chronic neuropathy and influencing factors in patients with colorectal cancer in iran. Asian Pac J Cancer Prev 2015;16:7603-6.
- Burakgazi AZ, Messersmith W, Vaidya D, Hauer P, Hoke A, Polydefkis M, et al. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology 2011;77:980-6.
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer 2008;44:1507-15.
- Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, et al. Clinical course of oxaliplatin-induced neuropathy: Results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 2015;33:3416-22.
- Argyriou AA, Velasco R, Briani C, Cavaletti G, Bruna J, Alberti P, et al. Peripheral neurotoxicity of oxaliplatin incombination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): A prospective evaluation of 150 colorectal cancer patients. Ann Oncol 2012;23:3116-22.
- Baek KK, Lee J, Park SH, Park JO, Park YS, Lim HY, et al. Oxaliplatin-induced chronic peripheral neurotoxicity: A prospective analysis in patients with colorectal cancer. Cancer Res Treat 2010;42:185-90.
- Pietrangeli A, Leandri M, Terzoli E, Jandolo B, Garufi C. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol 2006;56:13-6.
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC, et al. Long-term neuropathy after oxaliplatin treatment: Challenging the dictum of reversibility. Oncologist 2011;16:708-16.


 PDF
PDF  Views
Views  Share
Share

