Outcomes with Palliative Weekly Paclitaxel in Advanced, Recurrent, and Metastatic Esophageal Cancer - Real World Experience
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 46-51
DOI: DOI: 10.4103/ijmpo.ijmpo_39_17
Abstract
Background: In advanced esophageal cancer, combination chemotherapy regimens provide effective palliation but result in substantial toxicity. Aim: The aim of the study was to evaluate outcomes of recurrent and metastatic esophageal carcinoma treated with weekly paclitaxel. Objectives: The objective of the study was to determine the clinical and laboratory factors predicting response and affecting overall survival (OS) in patients receiving palliative chemotherapy for advanced esophageal/gastroesophageal cancer. Materials and Methods: Retrospective analysis of patients with advanced esophageal cancer, not amenable to definitive intent therapy that was treated with intravenous weekly paclitaxel was done. Progression-free survival (PFS) and OS were calculated with Kaplan Meir analysis while factors affecting outcome were subjected to log rank test and multivariate analysis. Results: Between September 2010 and October 2014, 350 patients were included in analysis. Median follow-up is 8 months. Median age was 55 years, with a male:female ratio of 2.4:1. Nearly 34.5% were mid esophageal and 51% were lower third and gastroesophageal junction tumors. Almost 58% of the tumors had squamous histology. Performance status was >2 in 25.4%. Almost 62% patients had received prior therapy. Median number of cycles of weekly paclitaxel delivered was 12 with median duration of 3 months. Nearly 51% of patients had improvement in dysphagia, with time to symptom improvement of 20 days. In 31% patients, feeding nasogastric tube could be removed. Overall response rate was 32% (complete remission, 2.5% + partial remission, 29.5%). Median PFS was 4.0 months (95% confidence interval [CI]: 3.6?4.3 months) and median OS was 10 months (95% CI: 8.5?11.4 months). Performance status and pretreatment albumin significantly affected OS. Conclusion:?Metronomic weekly paclitaxel chemotherapy provides good palliative benefit in advanced unresectable/metastatic esophageal cancer with minimal toxicity. Eastern Cooperative Oncology Group Performance Status (PS 0 and 1) and baseline serum albumin level >3.7 g/dl significantly improved survival.
Keywords
Advanced esophageal carcinoma - metronomic therapy - weekly paclitaxel
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: In advanced esophageal cancer, combination chemotherapy regimens provide effective palliation but result in substantial toxicity. Aim: The aim of the study was to evaluate outcomes of recurrent and metastatic esophageal carcinoma treated with weekly paclitaxel. Objectives: The objective of the study was to determine the clinical and laboratory factors predicting response and affecting overall survival (OS) in patients receiving palliative chemotherapy for advanced esophageal/gastroesophageal cancer. Materials and Methods: Retrospective analysis of patients with advanced esophageal cancer, not amenable to definitive intent therapy that was treated with intravenous weekly paclitaxel was done. Progression-free survival (PFS) and OS were calculated with Kaplan Meir analysis while factors affecting outcome were subjected to log rank test and multivariate analysis. Results:?Between September 2010 and October 2014, 350 patients were included in analysis. Median follow-up is 8 months. Median age was 55 years, with a male:female ratio of 2.4:1. Nearly 34.5% were mid esophageal and 51% were lower third and gastroesophageal junction tumors. Almost 58% of the tumors had squamous histology. Performance status was >2 in 25.4%. Almost 62% patients had received prior therapy. Median number of cycles of weekly paclitaxel delivered was 12 with median duration of 3 months. Nearly 51% of patients had improvement in dysphagia, with time to symptom improvement of 20 days. In 31% patients, feeding nasogastric tube could be removed. Overall response rate was 32% (complete remission, 2.5% + partial remission, 29.5%). Median PFS was 4.0 months (95% confidence interval [CI]: 3.6?4.3 months) and median OS was 10 months (95% CI: 8.5 11.4 months). Performance status and pretreatment albumin significantly affected OS. Conclusion: Metronomic weekly paclitaxel chemotherapy provides good palliative benefit in advanced unresectable/metastatic esophageal cancer with minimal toxicity. Eastern Cooperative Oncology Group Performance Status (PS 0 and 1) and baseline serum albumin level >3.7 g/dl significantly improved survival.
Keywords
Advanced esophageal carcinoma - metronomic therapy - weekly paclitaxel
Introduction
Esophageal cancer is one of the most aggressive cancers with dismal outcomes despite surgery, radiotherapy, and chemotherapy.[1] In patients with advanced unresectable or metastatic disease, the prognosis is grim with an estimated median survival of 6?7 months, regardless of whether the patient is treated with single agent chemotherapy or aggressive three-drug combination chemotherapy.[2],[3],[4] Taxanes have demonstrated promising activity in patients with esophageal cancer.[4] Paclitaxel has been used in various schedules, ranging from 24 h infusion to short 1 h infusion; however, shorter infusions have similar efficacy and lesser toxicity.[5] Metronomic weekly paclitaxel in smaller studies has shown modest response in unresectable and metastatic esophageal carcinoma. We present largest patient data from tertiary cancer center regarding experience with metronomic weekly paclitaxel in patients with advanced unresectable, metastatic, or recurrent esophageal and esophagogastric carcinomas.
Materials and Methods
We retrieved data of patients prospectively collected in our esophageal cancer database of recurrent, unresectable, and metastatic esophageal carcinoma registered between September 2010 and October 2014. Only patients who had confirmed histology and subsite available, received weekly paclitaxel as palliative chemotherapy, and having at least one response evaluation done as per Response Evaluation Criteria in Solid Tumor (RECIST, version 1.1, European Organisation for Research and Treatment of Cancer (EORTC), National Cancer Institute of United States, National Cancer Institute of Canada Clinical Trialist group, revised in 2008) were selected for final analysis. This study was approved by the Institutional Ethics Committee.
All patients were evaluated at baseline including history and physical examination, laboratory parameters, upper endoscopy (if indicated), and imaging studies. Patients were treated with paclitaxel at 80 mg/m 2 as a 1 h infusion given weekly with standard premedications including antihistamines, antiemetic, H2 antagonists, and steroids. Each dose of chemotherapy was considered as one cycle. If there was no evidence of hypersensitivity in the first few cycles of chemotherapy, steroids were omitted in subsequent cycles. Complete blood count was checked weekly and patients were evaluated by a physician weekly before chemotherapy. Serum biochemistry (including fasting blood glucose, liver functions, and renal functions) and serum electrolytes were checked once a month.
Response was calculated using standard response evaluation criteria in solid tumor (RECIST version 1.1) definitions, with the measurements including the maximum esophageal thickness added to other measurable lesions. Follow-up was taken from the case records, electronic medical records, and telephonic conversation with a patient or their relatives. Progression-free survival (PFS) was calculated from the date of receiving first dose of paclitaxel chemotherapy to the date of radiological progression, symptomatic deterioration in the absence of progressive disease (PD) on scan, start of next line of therapy due to any reason (logistic reasons, financial constraints, or unacceptable toxicity), or death from any cause. Overall survival (OS) was calculated from the date of first chemotherapy to the date of death from any cause. Toxicity was graded according to common terminology criteria for adverse events (Common terminology criteria for adverse events. CTC v 4.03 (National Cancer Institute Common Terminology Criteria for Adverse Events v4.0). Imaging studies were repeated approximately every eight cycles. Statistical Package for the Social Sciences version 18 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) was used for statistical analysis. For OS and PFS, Kaplan?Meier estimation curves were generated. Clinical factors and laboratory factors individually were tested with log-rank test. Those factors which prognosticated for OS were then tested in a Cox proportional Hazard method for multivariate analysis.
Results
Between September 2010 and October 2014, we selected data of 350 patients who were registered as recurrent/metastatic esophageal carcinoma in our prospectively maintained institutional database and received at least four doses of weekly paclitaxel with at least one response evaluation available. Median age at diagnosis is 55 years with a male:female ratio of 2.4:1 [Table 1]. Nearly 58% were squamous cell carcinoma whereas 42% had adenocarcinoma histology. Lower one-third was the most common subsite (51%,) followed by mid (35%) and upper esophagus (14%). Performance status was> 2 in 25.4%. Nearly 205 (59%) had baseline metastatic disease at presentation, whereas 218 (62%) had received previous therapy such as surgery, radiotherapy, or chemotherapy for definitive or palliative purpose. Among patients who received previous chemotherapy, 50% patients were platinum pretreated and 39% had received prior 3 weekly paclitaxel. Median number of cycles of weekly paclitaxel delivered was 12 with median total duration of 3 months. About 51% of patients had improvement in dysphagia on weekly paclitaxel, with a median time to symptom improvement of 20 days. In 31% of feeding tube-dependent patients, the nasogastric tube could be removed successfully.
|
Baseline factors |
Groups |
Values (%) |
|---|---|---|
|
*Hypertension, diabetic, ischemic heart disease, active asthma, COPD; #Surgery, radiotherapy, chemotherapy. COPD ? Chronic obstructive pulmonary disease |
||
|
Age (median 55 years) |
<55> |
211 (60.3) |
|
>55 |
139 (39.7) |
|
|
Sex |
Male |
247 (70.5) |
|
Female |
103 (29.4) |
|
|
Histology |
Squamous |
203 (58) |
|
Adenocarcinoma |
147 (42) |
|
|
Subsite |
Upper |
50 (14.3) |
|
Mid |
121 (34.6) |
|
|
Lower |
179 (51.1) |
|
|
Performance status |
0-1 |
261 (74.6) |
|
2 |
60 (17.1) |
|
|
3 |
29 (8.28) |
|
|
Comorbidities |
None |
224 (64) |
|
Any* |
126 (36) |
|
|
At presentation disease |
Metastatic |
205 (58.6) |
|
Non metastatic |
145 (41.4) |
|
|
Previous treatment |
Any# |
218 (62.3) |
|
None |
132 (37.7) |
|
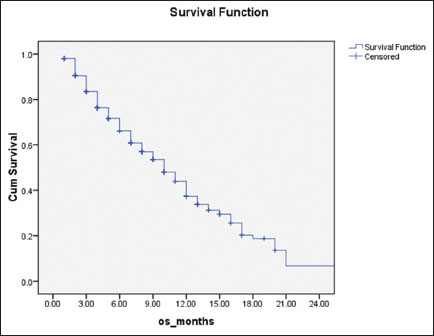
|?Figure.1Overall survival of patients (N = 350) treated with weekly paclitaxel
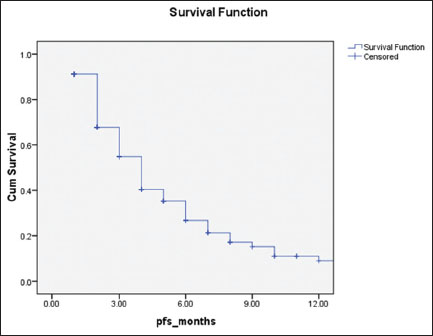
|?Figure.2Progression-free survival of patients (N = 360) treated with weekly paclitaxel
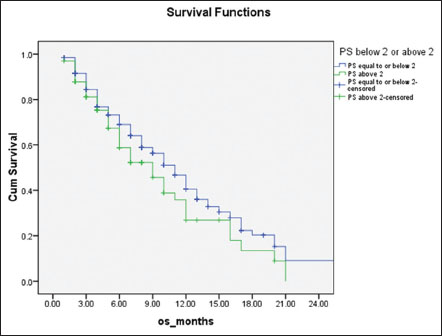
|?Figure.3Overall survival of patients stratified with performance status 0?1 (blue line) and 2 or more (green line), 11 months versus 9 months, respectively (P = 0.007)
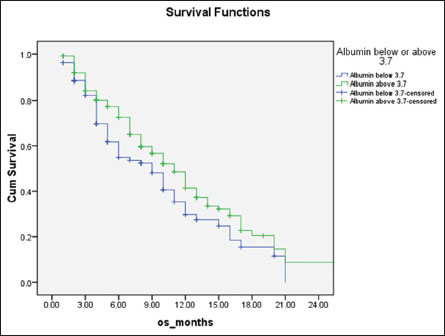
|?Figure.4Overall survival of patients stratified with serum albumin above (green line) and below (blue line) 3.7 mg/dL, 11 months versus 9 months, respectively (P = 0.03)
|
Factors |
Groups |
PFS (months) |
P |
OS (months) |
P |
|---|---|---|---|---|---|
|
PFS ? Progression-free survival; OS ? Overall survival |
|||||
|
Age (years) |
<55> |
4 |
0.9 |
11 |
0.16 |
|
>55 |
4 |
8 |
|||
|
Disease at |
Metastatic |
3 |
0.01 |
9 |
0.17 |
|
presentation |
Nonmetastatic |
5 |
11 |
||
|
Extent at |
Distant |
3 |
0.002 |
9 |
0.11 |
|
presentation |
Loco regional |
6 |
12 |
||
|
Hemoglobin |
<11> |
4 |
0.78 |
10 |
0.98 |
|
(g/dL) |
>11.4 |
4 |
10 |
||
|
Albumin |
<3> |
4 |
0.78 |
9 |
0.03 |
|
(g/dL) |
>3.7 |
4 |
11 |
||
|
PS |
0-1 |
6 |
0.007 |
11 |
0.007 |
|
>2 |
4 |
9 |
|||
|
Platinum |
Yes |
4 |
0.07 |
10 |
0.62 |
|
exposed |
No |
4 |
10 |
||
|
Previous |
Yes |
4 |
0.736 |
10 |
0.53 |
|
treatment |
No |
4 |
10 |
||
|
Characteristics |
Kato et al. |
Ilson et al. |
Noronha et al. |
Present series |
|---|---|---|---|---|
|
*Statistically significant; $Only squamous carcinoma and PS 0-1 were enrolled. NR ? Not reported; OS ? Overall survival; PFS ? Progression-free survival |
||||
|
Number of patients (n) |
53 |
95 |
51 |
350 |
|
Median age (years) |
65 |
59 |
56 |
55 |
|
Male/female |
6.6/1 |
12.5/1 |
2.9/1 |
2.4/1 |
|
Co-morbidities, n (%) |
None |
Excluded |
21 (41) |
126 (36) |
|
PS, n (%) |
||||
|
0-1 |
53 (100) |
PS 0-2 |
28 (55) |
261 (74.5) |
|
2 or more |
None |
NR |
23 (45) |
89 (25.5) |
|
Squamous versus (%) |
52 (98) |
32 (31) |
33 (65) |
203 (58) |
|
Adenocarcinoma (%) |
1 (2) |
63 (62) |
18 (35) |
147 (42) |
|
Previous platinum exposed (%) |
47/53 (88.6) |
22 (22) |
26 (51) |
175(50) |
|
Platinum naive (%) |
25 (49) |
175 (50) |
||
|
Median weekly paclitaxel doses |
10 |
12 |
11 |
12 |
|
Paclitaxel dose per week (mg/m2) |
100 |
80 |
80 |
80 |
|
Response rates (CR+PR) (%) |
43.5 (7.5+36) |
13 (0+13) |
49 (4+45) |
32 (2.5+29.5) |
|
Response platinum exposed versus naive (%) |
39.6 versus NR |
5 versus 15 |
35 versus 64* |
32 versus 37 |
|
PFS (median), months |
4.8 |
5.7 |
4.7 |
4 |
|
OS (median), months |
10.8 |
9 |
7.5 |
10 |
|
PFS |
||||
|
Squamous versus adenocarcinoma |
4.8 months$ |
NR |
5.9 versus 3.6 months* |
4 months versus 3 months |
|
PS 0-1 versus 2 or more |
4.8 months$ |
NR |
10.6 versus 5.4 months* |
6 months versus 4 months* |
|
Previous platinum versus none |
NR |
NR |
NR |
4 months versus 4 months |
|
Grade 3/4 neutropenia (%) |
52.8 |
5 |
9.8 |
17 |
|
Grade 3/4 leucopenia (%) |
45.3 |
- |
7.4 |
10 |
|
Febrile neutropenia (%) |
3.8 |
1 |
4 |
4 |
|
Grade 3/4 sensory neuropathy (%) |
5.7 |
3 |
2 |
5.8 |

|?Figure.1Overall survival of patients (N = 350) treated with weekly paclitaxel

|?Figure.2Progression-free survival of patients (N = 360) treated with weekly paclitaxel

|?Figure.3Overall survival of patients stratified with performance status 0?1 (blue line) and 2 or more (green line), 11 months versus 9 months, respectively (P = 0.007)

|?Figure.4Overall survival of patients stratified with serum albumin above (green line) and below (blue line) 3.7 mg/dL, 11 months versus 9 months, respectively (P = 0.03)
References
- oronha V, Patil V, Bhosale B, Joshi A, Purandare N, Prabhash K.?Metronomic weekly paclitaxel in advanced unresectable esophageal cancer. Indian J Cancer 2013; 50: 128-34
- an CutsemE, Moiseyenko VM, Tjulandin S, Majlis A, Constenla A, Boni C.?et al?Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006; 24: 4991-7
- ebb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK.?et al.?Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997; 15: 261-7
- oms MY, Gaast A, Siersema PD, Steyerberg EW, Kuipers EJ. Chemotherapy for metastatic carcinoma of the esophagus and gastro-esophageal junction. The Cochrane database of systematic reviews 2006:Cd004063
- illiams A, Bryant A. Short versus long duration infusions of paclitaxel for any advanced adenocarcinoma. The Cochrane database of systematic reviews 2011:Cd003911.
- alkan G, Mohandas KM.?Epidemiology of digestive cancers in India.I General principles and esophageal cancer. Indian J Gastroenterol 1997; 16: 98-102
- eole BB, Kurkure AP, Sunny L. Cancer survival in Mumbai (Bombay), India, 1992-1999?IARC scientific publications?2011;?133-42
- 8?Gr?nberger B, Raderer M, Schmidinger M, Hejna M.?Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res 2007; 27: 2705-14
- hau I, Norman AR, Cunningham D, Oates J, Hawkins R, Iveson T.?et al.?The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma ? Individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol 2009; 20: 885-91
- Wang K, Johnson A, Ali SM, Klempner SJ, Bekaii-Saab T, Vacirca JL.?et al.?Comprehensive genomic profiling of advanced esophageal squamous cell carcinomas and esophageal adenocarcinomas reveals similarities and differences. Oncologist 2015; 20: 1132-9
- Ilson DH.?Docetaxel, cisplatin, and fluorouracil in gastric cancer: Does the punishment fit the crime?. J Clin Oncol 2007; 25: 3188-90
- Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I.?et al.?A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 2004; 15: 955-9
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE.?Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24: 2903-9
- Ilson DH, Wadleigh RG, Leichman LP, Kelsen DP.?Paclitaxel given by a weekly 1-h infusion in advanced esophageal cancer. Ann Oncol 2007; 18: 898-902
- Kato K, Tahara M, Hironaka S, Muro K, Takiuchi H, Hamamoto Y.?et al.?A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol 2011; 67: 1265-72
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M.?Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993; 72: 37-41
- Pyrh?nen S, Kuitunen T, Nyandoto P, Kouri M.?Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995; 71: 587-91
- Glimelius B, Ekstr?m K, Hoffman K, Graf W, Sj?d?n PO, Haglund U.?et al?Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8: 163-8
- Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K.?et al.?Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer-a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). European journal of cancer (Oxford, England: 1990) 2011; 47: 2306-14
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J. et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010:Cd004064
- Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC.?et al.?Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012; 30: 1513-8
- Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K.?et al.?Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer ? A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011; 47: 2306-14
- Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J.?et al.?Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol 2014; 15: 78-86
- Janowitz T, Thuss-Patience P, Marshall A, Kang JH, Connell C, Cook N.?et al.?Chemotherapy vs supportive care alone for relapsed gastric, gastroesophageal junction, and oesophageal adenocarcinoma: A meta-analysis of patient-level data. Br J Cancer 2016; 114: 381-7
- Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N.?et al.?Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: A randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer 2014; 50: 1437-45
- Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M.?et al.?Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer 2015; 51: 808-16
- Sym SJ, Hong J, Park J, Cho EK, Lee JH, Park YH.?et al.?A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol 2013; 71: 481-8


 PDF
PDF  Views
Views  Share
Share

