Outcomes with Methotrexate-Free Dyad Chemotherapy in Osteosarcoma Patients: Audit from a Resource-Limited Setting
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(02): 191-199
DOI: DOI: 10.1055/s-0044-1795133
Abstract
Introduction Multiagent chemotherapy forms the backbone for the management of osteosarcoma. The globally accepted chemotherapy regimens for osteosarcoma include a combination of Adriamycin, cisplatin, and high-dose methotrexate (HDMTX). However, non-HDMTX regimens are predominantly used in India, secondary to patient profile, toxicity, administration, logistics, and financial constraints. We present our outcomes with a two-drug dyad chemotherapy consisting of Adriamycin and cisplatin in a resource-limited setting.
Objective To determine the disease free and overall survival of osteosarcoma patients and to evaluate the prognostic factors affecting OS for patients with localized disease.
Material and Methods The study was a record-based analysis of all osteosarcoma patients presenting at a tertiary care referral center during the period from 2010 to 2019. A total of 127 patients of osteosarcoma were identified, who were evaluated for their demographic and clinical profile, while treatment details and outcomes were evaluated in 123 patients as disease-free survival (DFS) and overall survival (OS). Univariate and multivariate analysis was done for factors influencing OS.
Results The median age at presentation was 18 years and extremities were the most common site of presentation. Localized disease (LD) was seen in 102 (80%) patients, while 25 (20%) patients had metastatic disease (MD). Overall, 83 (84%) patients with LD underwent surgery, of whom 65 (78%) underwent limb salvage surgery, while 18 (22%) underwent amputation. Only 72 (73%) patients completed the planned six cycles of chemotherapy. At a median follow-up of 50.4 (range: 1–166.3) months, the 5-year OS for patients with LD and the entire cohort was 53 and 43%, respectively. For patients with MD, the 1- and 2-year OS were 41 and 7%, respectively. The 3- and 5-year DFS for patients with LD was 41 and 35%, respectively. Primary tumor measuring less than 12 cm (p = 0.03) and patients undergoing surgery (p = 0.003) were found to be statistically significant for improved OS on univariate analysis but not on multivariate analysis.
Conclusion The two-drug dyad chemotherapy was well tolerated with manageable toxicity. The outcomes were comparable with Indian studies using non-HDMTX regimens that report a 5-year survival of within 50 to 60%, but were inferior to global outcomes and the dose-dense OGS-12 protocol used in India. Raising awareness for early diagnosis, improving the nutritional status, incorporation of sequential third drug (ifosfamide), use of dose-intensive regimens for selected patients, and increasing compliance to treatment may further help improve the outcomes.
Keywords
osteosarcoma - pediatric tumor - resource-limited setting - chemotherapy - non-high-dose methotrexate - survival
Authors' Contributions
All the authors read and approved the manuscript and contributed to it.
N.G. contributed to the concept, data collection, data analysis, and preparation and finalization of the draft. K.D. contributed to the supervision of data collection and revision of the draft. S.K.G. contributed to the concept and revision of the draft. A.K.P. contributed to the concept and supervision of data collection. A.A. contributed to data collection and data analysis.
Patient Consent
None declared.
Publication History
Article published online:
13 December 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Obstacles facing gastrointestinal endoscopy in a resource-limited settingA Ebigbo, Zeitschrift für Gastroenterologie, 2018
- Measurement of the Optic Nerve in a Resource-Limited SettingNicola Rosa, Journal of Neurosciences in Rural Practice, 2017
- Measurement of the Optic Nerve in a Resource-Limited SettingNicola Rosa, Journal of Neurosciences in Rural Practice, 2017
- Evaluation of trans burr hole ultrasonography usefulness in a resource-limited settingV. de P. Djientcheu, Indian Journal of Neurosurgery, 2013
- Evaluation of trans burr hole ultrasonography usefulness in a resource-limited settingV. de P. Djientcheu, Indian Journal of Neurosurgery, 2013
- Management of adolescents and adults with febrile illness in resource limited areas<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Methotrexate dosage in patients aged over 50 with psoriasis.<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Patients' and general practitioners' satisfaction with information given on discharge from hospital: audit of a new information card.<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Doctors’ view of care pathway for dying patients clashes with audit findings<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Severe myalgia from an interaction between treatments with pantoprazole and methotrexate<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Introduction Multiagent chemotherapy forms the backbone for the management of osteosarcoma. The globally accepted chemotherapy regimens for osteosarcoma include a combination of Adriamycin, cisplatin, and high-dose methotrexate (HDMTX). However, non-HDMTX regimens are predominantly used in India, secondary to patient profile, toxicity, administration, logistics, and financial constraints. We present our outcomes with a two-drug dyad chemotherapy consisting of Adriamycin and cisplatin in a resource-limited setting.
Objective To determine the disease free and overall survival of osteosarcoma patients and to evaluate the prognostic factors affecting OS for patients with localized disease.
Material and Methods The study was a record-based analysis of all osteosarcoma patients presenting at a tertiary care referral center during the period from 2010 to 2019. A total of 127 patients of osteosarcoma were identified, who were evaluated for their demographic and clinical profile, while treatment details and outcomes were evaluated in 123 patients as disease-free survival (DFS) and overall survival (OS). Univariate and multivariate analysis was done for factors influencing OS.
Results The median age at presentation was 18 years and extremities were the most common site of presentation. Localized disease (LD) was seen in 102 (80%) patients, while 25 (20%) patients had metastatic disease (MD). Overall, 83 (84%) patients with LD underwent surgery, of whom 65 (78%) underwent limb salvage surgery, while 18 (22%) underwent amputation. Only 72 (73%) patients completed the planned six cycles of chemotherapy. At a median follow-up of 50.4 (range: 1–166.3) months, the 5-year OS for patients with LD and the entire cohort was 53 and 43%, respectively. For patients with MD, the 1- and 2-year OS were 41 and 7%, respectively. The 3- and 5-year DFS for patients with LD was 41 and 35%, respectively. Primary tumor measuring less than 12 cm (p = 0.03) and patients undergoing surgery (p = 0.003) were found to be statistically significant for improved OS on univariate analysis but not on multivariate analysis.
Conclusion The two-drug dyad chemotherapy was well tolerated with manageable toxicity. The outcomes were comparable with Indian studies using non-HDMTX regimens that report a 5-year survival of within 50 to 60%, but were inferior to global outcomes and the dose-dense OGS-12 protocol used in India. Raising awareness for early diagnosis, improving the nutritional status, incorporation of sequential third drug (ifosfamide), use of dose-intensive regimens for selected patients, and increasing compliance to treatment may further help improve the outcomes.
Keywords
osteosarcoma - pediatric tumor - resource-limited setting - chemotherapy - non-high-dose methotrexate - survival
Introduction
Osteosarcoma is a rare bone tumor with an annual incidence of 0.3 per 100,000.[1] Nevertheless, in spite of its rarity, it is the most common primary bone tumor.[1] [2] Osteosarcoma mainly affects children and adolescents.[2] [3] The majority of osteosarcomas arise in extremities, and the lung is the most common site of metastases, followed by bones.[1] [4]
Magnetic resonance imaging (MRI) is considered the investigation of choice for evaluation of primary bone tumor. Computed tomography (CT) scans of the chest and bone are preferred to exclude metastatic disease (MD).[2] [4] Standard treatment for osteosarcoma consists of induction chemotherapy, followed by surgery and subsequent completion of adjuvant chemotherapy. Radiotherapy has limited role in view of relative radioresistant nature of the tumor.[4] [5]
In developed countries, the overall survival (OS) for patients with localized disease (LD) and MD is around 60 to 75%. and 30 to 40%, respectively.[6] [7] Standard chemotherapy regimens in osteosarcoma include a dyad of chemotherapy consisting of cisplatin and doxorubicin or the MAP regimen: doxorubicin/cisplatin/high-dose methotrexate (HDMTX).[4] [5] [6] While HDMTX is the standard of care for the European and American patients, non-HDMTX regimens are predominantly used in developing countries. The hesitancy to use HDMTX regimens in developing countries is because patients present with poor performance status, costs, and excess toxicity. These patients undergo treatment in medical institutions with limited infrastructure in terms of indoor capacity. They also need constant drug-level monitoring and supportive care.[8] [9] Non-HDMTX-based regimens are the most commonly used regimens in the majority of the cancer centers in India for high-grade osteosarcoma.[3] [10] [11]
Published data on osteosarcoma from India are very limited; hence, the exact magnitude and disease trend are not properly understood. There have been a few Indian studies in recent years, which report the 5-year survival rates as somewhat inferior to the world literature.[2] [10] [11] We present here the clinicodemographic profile, treatment patterns, and outcomes in terms of DFS and OS for osteosarcoma patients managed with dyad chemotherapy with Adriamycin and cisplatin (AC) in a resource-limited setting where patients generally present late with large tumors and poor performance status.
Materials and Methods
Study Design
This is a retrospective observational study that involves a record-based analysis of all osteosarcoma patients diagnosed and treated at a tertiary care referral center during the period from 2010 to 2019.
Sample Size
A total of 127 histopathologically proven patients of osteosarcoma were identified, who were evaluated for their demographic and clinical profile. One hundred and twenty-three patients who reported for treatment were evaluated for treatment details, recurrence patterns, and survival outcomes.
Inclusion and Exclusion Criteria
All biopsy-proven patients of osteosarcoma who underwent treatment at the tertiary care referral center during the period from 2010 to 2019 were included in the analysis. Patients who did not have a histopathology confirmation from the institutional pathology department or who did not receive treatment at our center were excluded from the analysis.
Primary and Secondary Outcomes
Primary outcomes included the following:
Evaluation of the disease-free survival (DFS) and OS.
Evaluation of the demographic and clinical profile of the osteosarcoma patients.
Secondary outcomes included evaluation of the prognostic factors affecting OS for patients with LD.
Study Setting
Data were analyzed for the demographic profile including age at presentation, gender, baseline body mass index (BMI) and hemoglobin levels, rural or urban residence, and any preexisting morbidities or addiction. The clinical profile was evaluated for symptoms at presentation, duration of symptoms before initiating treatment, tumor site, laterality, radiological investigation done for the primary site and MD, maximum size of the primary tumor, and the presence of LD or MD.
Treatment for LD or for patients with curative intent consisted of delivering three to four cycles of neoadjuvant chemotherapy (NACT) followed by surgery, which was followed by adjuvant consolidation chemotherapy. As per our institutional protocol, three to four cycles of dyad chemotherapy were delivered in the neoadjuvant setting consisting of AC regimen[4] as follows.
Doxorubicin 25 mg/m2/d IV over 2 hours (days 1–3), cisplatin 100 mg/m2 IV over 3 hours (day 1), and cycles repeated every 3 weeks. Prophylactic growth factors were not used. The details of NACT and adjuvant chemotherapy delivered in terms of regimen, the number of cycles, toxicity, and timing with respect to local treatment were analyzed. Adjuvant radiotherapy was added for selected patients, predominantly for positive margins. Surgery and radiation details were also evaluated.
Response to NACT was assessed clinically and radiologically and decisions for surgery were taken. Histological evaluation for response to chemotherapy and extent of tumor necrosis was assessed using the Huvos grading system.[12] In the initial years when the Huvos grading was not done universally, many patients did not have the information available in the histopathology reports. Adjuvant chemotherapy was given with the aim to complete a total of six cycles.[4]
Management including chemotherapy protocols for patients with recurrent or MD were selected from the recommended options from standard treatment guidelines.[4] These protocols were individualized based on disease burden, site of metastases, general condition of the patient, and family decision. Recurrence patterns, treatment for recurrence, and MD were also analyzed. Outcomes were evaluated in terms of DFS and OS. OS was calculated from the date of registration in the department to death from any cause, while DFS was calculated from the date of registration to the first event (local recurrence, metastases, or death from any cause). Prognostic factors affecting OS for patients with LD were assessed.
Statistical Analysis
Statistical analysis was done using Statistical Package for Social Sciences version 17 (IBM Inc., Chicago, IL, United States). Descriptive statistics were used for demographic and clinical parameters and treatment modalities, and were reported as median and percentages. OS and progression-free survival were estimated according to the Kaplan–Meier method, stratified by the LD and MD. Univariate and multivariate (Cox proportional hazards regression model) analyses were used to assess the factors influencing OS in patients with LD. Multivariate analysis was performed on the factors that were found to be significant on univariate analysis. A p value of less than 0.05 was considered significant. Age of patient (>21 years), gender, duration of presenting symptoms (>6 months), primary site (lower extremity vs. upper extremity), primary tumor size (<12>
Ethics
Waiver was obtained from the institutional ethics committee (reference number GMCH/IEC/2024/1341) as this was a record-based analysis and did not involve any patient interaction or intervention.
Results
A total of 127 patients were evaluated for demographic and clinical profile. Four patients did not report for treatment. The remaining 123 patients were evaluated for treatment details, recurrence pattern, and outcomes.
Demography
In our registry, the median age at presentation was 18 years. The majority of patients had poor nutritional status as reflected by the BMI and baseline hemoglobin. Seventy-seven (61%) patients had a BMI less than 18.5 and 25 (20%) patients had baseline hemoglobin less than 10 g/dL. Details of age and gender distribution, BMI, residence, marital status, comorbidities, and addiction habits are listed in [Table 1].
|
Parameter |
n = 127 (%) |
|---|---|
|
Age (y) |
|
|
0–10 |
4 (3.2) |
|
11–20 |
83 (65.4) |
|
21–30 |
27 (21.3) |
|
>30 |
13 (10.2) |
|
Median age (y) |
18 (8–63) |
|
Sex |
|
|
Male |
86 (67.7) |
|
Female |
41 (32.3) |
|
Median hemoglobin (g/dL), n (range) |
11.8 (6.8–15.6) |
|
Median body mass index (BMI) |
17.3 (4.8–31.8) |
|
<18> |
77 (60.6) |
|
18.6–22.9 |
39 (30.7) |
|
>23 |
11 (8.7) |
|
Residence |
|
|
Urban |
44 (34.6) |
|
Rural |
83 (65.4) |
|
Marital status |
|
|
Single |
110 (86.6) |
|
Married |
17(13.4) |
|
Morbidity |
|
|
Epilepsy |
4(3.2) |
|
Tuberculosis |
4 (3.2) |
|
CAD |
2(1.6) |
|
None |
119 (93.7) |
|
Addiction |
|
|
Tobacco |
6 (4.7) |
|
Alcohol |
4 (3.2) |
|
None |
119 (93.7) |
|
Parameter |
n = 127 (%) |
|---|---|
|
Presenting symptom |
|
|
Pain |
71 (56) |
|
Swelling |
94 (74) |
|
Restricted movement |
28 (22) |
|
History of trauma |
14 (11) |
|
Pathological fracture at presentation |
9 (7.1) |
|
Duration of symptoms before reporting (mo) |
|
|
<3> |
74 (58.3) |
|
3–6 |
30 (23.6) |
|
6–12 |
15(11.8) |
|
>12 |
8(6.3) |
|
Site |
|
|
Extremity |
122 (96) |
|
Pelvis |
2 (1.6) |
|
Face (mandible) |
1 (0.8) |
|
Soft tissue/extraskeletal |
2 (1.6) |
|
Common extremity subsite |
|
|
Femur |
59 (46.5) |
|
Tibia |
39 (30.7) |
|
Humerus |
19 (15) |
|
Fibula |
4 (3.2) |
|
Laterality |
|
|
Left |
72 (56.7) |
|
Right |
55 (43.3) |
|
Radiological investigation for primary |
|
|
MRI |
117 (92.1) |
|
CT scan |
10 (7.9) |
|
Radiological size of primary |
|
|
<8> |
38 (30) |
|
8–12 cm |
54 (42.5) |
|
>12 cm |
35 (27.6) |
|
Radiology consistent with OS |
64 (50.4) |
|
Radiological investigation for metastatic disease |
|
|
CXR |
9 (7) |
|
CT chest |
112 (88.2) |
|
PET scan |
6 (4.7) |
|
Disease at presentation |
|
|
Localized |
102 (80.3) |
|
Metastatic |
25 (19.7) |
|
Bone marrow positive |
10/38 (7.9) |
|
Parameter |
n (%) |
|---|---|
|
Neoadjuvant chemotherapy |
89 (89.9) |
|
Median number of cycles |
3 (1–6) |
|
AC |
89 (100) |
|
Surgery |
83 (83.8) |
|
Limb salvage surgery |
65 (78) |
|
Amputation |
18 (22) |
|
Margin positive |
4 (4.8) |
|
Adjuvant radiotherapy |
4 |
|
Dose: 45–54 Gy |
4 |
|
Tumor necrosis |
51 |
|
Grade 1 |
14 (27.5) |
|
Grade 2 |
9 (17.6) |
|
Grade 3 |
8 (15.7) |
|
Grade 4 |
12 (23.5) |
|
Adjuvant chemotherapy |
76 (76.8) |
|
Median number of cycles |
3 (0–6) |
|
Adriamycin/cisplatin |
65 (85.5) |
|
Ifosfamide/etoposide |
11 (14.5) |
|
Chemotherapy completed: 6 cycles |
|
|
Yes |
72 (72.7) |
|
No |
27 (27.3) |
|
Toxicity grade 3/4 |
|
|
Anemia |
18 (18.1) |
|
Neutropenia |
26 (26.3) |
|
sepsis and shock |
2 (2) |
|
Vomiting |
4 (4) |
|
Renal failure |
1 (1) |
|
PD on 4 wk after adjuvant chemotherapy |
10 (10.1) |
|
Parameter |
n (%) |
|---|---|
|
Treatment for relapse/progressive disease ( n = 39) |
|
|
Site of recurrence/progression |
|
|
Bone |
8 (20.5) |
|
Lungs |
22 (56.4) |
|
Lungs and bones |
6 (15.4) |
|
Lungs and brain |
3 (7.7) |
|
Local site |
11 (28.2) |
|
Treatment |
|
|
Chemotherapy |
28 (71.8) |
|
Median number of cycles |
1 (1–6) |
|
Gemcitabine/docetaxel |
3 |
|
Adriamycin/cisplatin |
3 |
|
Ifosfamide/etoposide |
15 |
|
Gemcitabine/cisplatin |
3 |
|
Gemcitabine |
2 |
|
Oral metronomic |
2 |
|
Surgery |
4 (10.3) |
|
Amputation |
2 |
|
Local resection |
2 |
|
Radiotherapy |
7 (18) |
|
20–30 Gy |
5 |
|
56–60 Gy |
2 |
|
Treatment for metastatic disease at presentation ( n = 24) |
|
|
Sites of metastases |
|
|
Lungs |
22 (91.7) |
|
Lungs and bones |
2 (8.3) |
|
First-line chemotherapy |
24 (100) |
|
Median number of cycles |
3 (1–6) |
|
Adriamycin/cisplatin |
20 |
|
Adriamycin/cisplatin/ifosfamide |
5 |
|
Second-line chemotherapy |
7 (29.2) |
|
Median number of cycles |
3 (1–6) |
|
Ifosfamide/etoposide |
2 |
|
Docetaxel/gemcitabine |
4 |
|
Pazopanib |
1 |
|
Surgery |
13 (54.2) |
|
Amputation |
7 |
|
Local resection |
5 |
|
Radiotherapy (20–30 Gy) |
3 (12.5) |
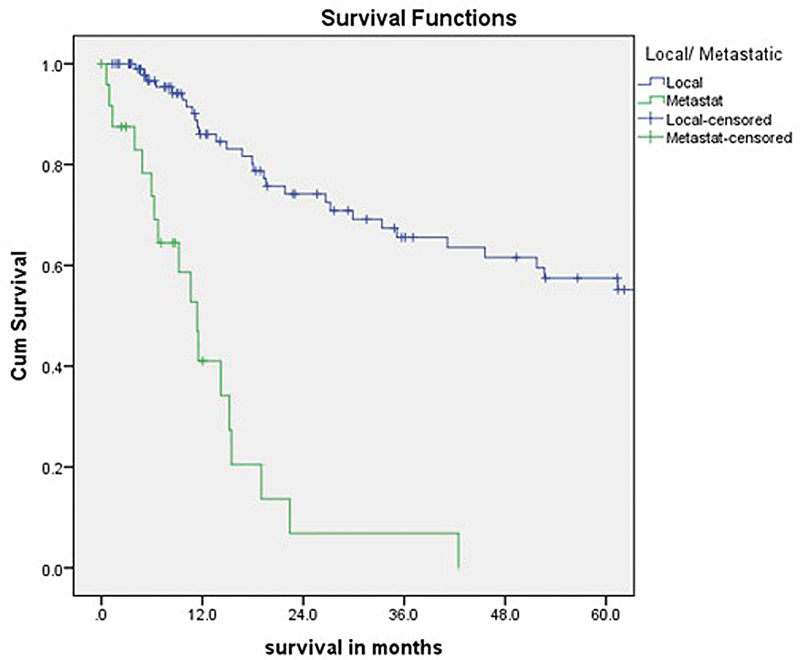
Fig 1: 5-Year Survival for patients with Localized Disease (Local) and Metastatic Disease (Metastat)
|
Variable |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
|
HR |
CI |
p value |
HR |
CI |
p value |
|
|
Age >21 y |
0.82 |
0.39–1.74 |
0.61 |
|||
|
Gender (male) |
1.72 |
0.77–3.83 |
0.18 |
|||
|
Duration of presenting symptoms >6 mo |
0.88 |
0.39–1.96 |
0.75 |
|||
|
Primary site (lower extremity vs. upper extremity) |
2.02 |
0.82–4.95 |
0.12 |
|||
|
Primary tumor size <12> |
0.28 |
0.87–0.9 |
0.03 |
2.22 |
0.79–6.21 |
0.12 |
|
No. of chemotherapy cycles: ≥6 |
0.54 |
0.25–1.14 |
0.10 |
|||
|
Underwent surgery |
0.25 |
0.10–0.65 |
0.003 |
0.26 |
0.06–1.15 |
0.075 |
|
Necrosis grade 4 vs. 1 |
0.24 |
0.03–2.04 |
0.19 |
|||
|
Local vs. metastatic disease |
3.87 |
2.11–7.09 |
0.001 |
|||
References
- Strauss SJ, Frezza AM, Abecassis N. et al; ESMO Guidelines Committee, EURACAN, GENTURIS and ERN PaedCan. Electronic address: clinicalguidelines@esmo.org. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2021; 32 (12) 1520-1536
- Blay JY, Palmerini E, Bollard J. et al. SELNET clinical practice guidelines for bone sarcoma. Crit Rev Oncol Hematol 2022; 174: 103685
- Bajpai J, Chandrasekharan A, Simha V. et al. Osteosarcoma journey over two decades in India: small steps, big changes. Pediatr Blood Cancer 2019; 66 (09) e27877
-
NCCN. NCCN clinical practice guidelines in oncology: Bone Cancer. Accessed May 1, 2024 at: https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf
-
- Bernthal NM, Federman N, Eilber FR. et al. Long-term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high-grade, operable osteosarcoma. Cancer 2012; 118 (23) 5888-5893
- Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol 2010; 19 (04) 193-199
- Gupta N, Chugh Y, Prinja S. Bridging the cancer care gap and inequities in radiation treatment in India: a narrative review. Cancer Res Stat Treat 2023; 6: 554-561
- Graf N, Winkler K, Betlemovic M, Fuchs N, Bode U. Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 1994; 12 (07) 1443-1451
- Nataraj V, Batra A, Rastogi S. et al. Developing a prognostic model for patients with localized osteosarcoma treated with uniform chemotherapy protocol without high dose methotrexate: a single-center experience of 237 patients. J Surg Oncol 2015; 112 (06) 662-668
-
- Huvos AG. Bone Tumors: Diagnosis, Treatment, and Prognosis. New York, NY: Saunders; 1991
- Prinja S, Dixit J, Gupta N. et al. Financial toxicity of cancer treatment in India: towards closing the cancer care gap. Front Public Health 2023; 11: 1065737
- Gupta N, Takkar N. Fertility preservation in women undergoing treatment for malignancies: a narrative review. Int J Reprod Contracept Obstet Gynecol 2024; 13: 776-783
- Smeland S, Bielack SS, Whelan J. et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 2019; 109: 36-50
- Bajpai J, Jaffe N. Perspectives of the role of chemotherapy in the management of osteosarcoma. J Cancer Ther 2012; 3: 1191-1203
- Lewis IJ, Nooij MA, Whelan J. et al; MRC BO06 and EORTC 80931 collaborators, European Osteosarcoma Intergroup. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007; 99 (02) 112-128
- Anninga JK, Gelderblom H, Fiocco M. et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand?. Eur J Cancer 2011; 47 (16) 2431-2445
-
- Gupta N, Dimri K, Garg SK, Arora A, Pandey AK. Real world data of Ewing sarcoma from a resource-limited setting with poor compliance to treatment leading to poor outcomes. ecancer 2024; 18: 1801
- Smeland S, Müller C, Alvegard TA. et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer 2003; 39 (04) 488-494
- Meyers PA, Schwartz CL, Krailo MD. et al; Children's Oncology Group. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children's Oncology Group. J Clin Oncol 2008; 26 (04) 633-638
- Marina NM, Smeland S, Bielack SS. et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 2016; 17 (10) 1396-1408
-
- Gupta N, Pandey A, Dimri K, Prinja S. Epidemiological profile of retinoblastoma in North India: implications for primary care and family physicians. J Family Med Prim Care 2020; 9 (06) 2843-2848
-
- Gupta N, Pandey AK, Dimri K, Jyani G, Goyal A, Prinja S. Health-related quality of life among breast cancer patients in India. Support Care Cancer 2022; 30 (12) 9983-9990
- DeLaney TF, Park L, Goldberg SI. et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 2005; 61 (02) 492-498
- Ferrari S, Briccoli A, Mercuri M. et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol 2003; 21 (04) 710-715
- Prinja S, Gupta N. Value-based pricing for cancer drugs in India. Cancer Res Stat Treat 2021; 4: 559-560
- Bielack SS, Kempf-Bielack B, Branscheid D. et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol 2009; 27 (04) 557-565
- Dixit J, Gupta N, Kataki A. et al. Health-related quality of life and its determinants among cancer patients: evidence from 12,148 patients of Indian database. Health Qual Life Outcomes 2024; 22 (01) 26
- Bajpai J, Chandrasekharan A, Talreja V. et al. Outcomes in non-metastatic treatment naive extremity osteosarcoma patients treated with a novel non-high dosemethotrexate-based, dose-dense combination chemotherapy regimen “OGS-12.”. Eur J Cancer 2017; 85: 49-58
- Vasquez L, Tarrillo F, Oscanoa M. et al. Analysis of prognostic factors in high-grade osteosarcoma of the extremities in children: a 15-year single-institution experience. Front Oncol 2016; 6: 22
- Whelan JS, Jinks RC, McTiernan A. et al. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol 2012; 23 (06) 1607-1616
Address for correspondence
Publication History
Article published online:
13 December 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Obstacles facing gastrointestinal endoscopy in a resource-limited settingA Ebigbo, Zeitschrift für Gastroenterologie, 2018
- Measurement of the Optic Nerve in a Resource-Limited SettingNicola Rosa, Journal of Neurosciences in Rural Practice, 2017
- Measurement of the Optic Nerve in a Resource-Limited SettingNicola Rosa, Journal of Neurosciences in Rural Practice, 2017
- Evaluation of trans burr hole ultrasonography usefulness in a resource-limited settingV. de P. Djientcheu, Indian Journal of Neurosurgery, 2013
- Evaluation of trans burr hole ultrasonography usefulness in a resource-limited settingV. de P. Djientcheu, Indian Journal of Neurosurgery, 2013
- Association between homologous recombination deficiency and outcomes with platinum and platinum-free chemotherapy in patients with triple-negative breast cancer<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Clinical outcomes of second-line chemotherapy in patients with advanced pancreatic adenocarcinoma: a real-world study<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Chemotherapy-free radiotherapy combined with immune checkpoint inhibitors: a new regimen for locally advanced non-small cell lung cancer?<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Chemotherapy-free radiotherapy combined with immune checkpoint inhibitors: a new regimen for locally advanced non-small cell lung cancer?<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Integrated strategies for chemotherapy cycles in nasopharyngeal carcinoma patients: Real-world data from two epidemic centers guiding decision-making<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

Fig 1: 5-Year Survival for patients with Localized Disease (Local) and Metastatic Disease (Metastat)
References
- Strauss SJ, Frezza AM, Abecassis N. et al; ESMO Guidelines Committee, EURACAN, GENTURIS and ERN PaedCan. Electronic address: clinicalguidelines@esmo.org. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2021; 32 (12) 1520-1536
- Blay JY, Palmerini E, Bollard J. et al. SELNET clinical practice guidelines for bone sarcoma. Crit Rev Oncol Hematol 2022; 174: 103685
- Bajpai J, Chandrasekharan A, Simha V. et al. Osteosarcoma journey over two decades in India: small steps, big changes. Pediatr Blood Cancer 2019; 66 (09) e27877
-
NCCN. NCCN clinical practice guidelines in oncology: Bone Cancer. Accessed May 1, 2024 at: https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf
-
- Bernthal NM, Federman N, Eilber FR. et al. Long-term results (>25 years) of a randomized, prospective clinical trial evaluating chemotherapy in patients with high-grade, operable osteosarcoma. Cancer 2012; 118 (23) 5888-5893
- Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol 2010; 19 (04) 193-199
- Gupta N, Chugh Y, Prinja S. Bridging the cancer care gap and inequities in radiation treatment in India: a narrative review. Cancer Res Stat Treat 2023; 6: 554-561
- Graf N, Winkler K, Betlemovic M, Fuchs N, Bode U. Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 1994; 12 (07) 1443-1451
- Nataraj V, Batra A, Rastogi S. et al. Developing a prognostic model for patients with localized osteosarcoma treated with uniform chemotherapy protocol without high dose methotrexate: a single-center experience of 237 patients. J Surg Oncol 2015; 112 (06) 662-668
-
- Huvos AG. Bone Tumors: Diagnosis, Treatment, and Prognosis. New York, NY: Saunders; 1991
- Prinja S, Dixit J, Gupta N. et al. Financial toxicity of cancer treatment in India: towards closing the cancer care gap. Front Public Health 2023; 11: 1065737
- Gupta N, Takkar N. Fertility preservation in women undergoing treatment for malignancies: a narrative review. Int J Reprod Contracept Obstet Gynecol 2024; 13: 776-783
- Smeland S, Bielack SS, Whelan J. et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 2019; 109: 36-50
- Bajpai J, Jaffe N. Perspectives of the role of chemotherapy in the management of osteosarcoma. J Cancer Ther 2012; 3: 1191-1203
- Lewis IJ, Nooij MA, Whelan J. et al; MRC BO06 and EORTC 80931 collaborators, European Osteosarcoma Intergroup. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007; 99 (02) 112-128
- Anninga JK, Gelderblom H, Fiocco M. et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand?. Eur J Cancer 2011; 47 (16) 2431-2445
-
- Gupta N, Dimri K, Garg SK, Arora A, Pandey AK. Real world data of Ewing sarcoma from a resource-limited setting with poor compliance to treatment leading to poor outcomes. ecancer 2024; 18: 1801
- Smeland S, Müller C, Alvegard TA. et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer 2003; 39 (04) 488-494
- Meyers PA, Schwartz CL, Krailo MD. et al; Children's Oncology Group. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children's Oncology Group. J Clin Oncol 2008; 26 (04) 633-638
- Marina NM, Smeland S, Bielack SS. et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 2016; 17 (10) 1396-1408
-
- Gupta N, Pandey A, Dimri K, Prinja S. Epidemiological profile of retinoblastoma in North India: implications for primary care and family physicians. J Family Med Prim Care 2020; 9 (06) 2843-2848
-
- Gupta N, Pandey AK, Dimri K, Jyani G, Goyal A, Prinja S. Health-related quality of life among breast cancer patients in India. Support Care Cancer 2022; 30 (12) 9983-9990
- DeLaney TF, Park L, Goldberg SI. et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 2005; 61 (02) 492-498
- Ferrari S, Briccoli A, Mercuri M. et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol 2003; 21 (04) 710-715
- Prinja S, Gupta N. Value-based pricing for cancer drugs in India. Cancer Res Stat Treat 2021; 4: 559-560
- Bielack SS, Kempf-Bielack B, Branscheid D. et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol 2009; 27 (04) 557-565
- Dixit J, Gupta N, Kataki A. et al. Health-related quality of life and its determinants among cancer patients: evidence from 12,148 patients of Indian database. Health Qual Life Outcomes 2024; 22 (01) 26
- Bajpai J, Chandrasekharan A, Talreja V. et al. Outcomes in non-metastatic treatment naive extremity osteosarcoma patients treated with a novel non-high dosemethotrexate-based, dose-dense combination chemotherapy regimen “OGS-12.”. Eur J Cancer 2017; 85: 49-58
- Vasquez L, Tarrillo F, Oscanoa M. et al. Analysis of prognostic factors in high-grade osteosarcoma of the extremities in children: a 15-year single-institution experience. Front Oncol 2016; 6: 22
- Whelan JS, Jinks RC, McTiernan A. et al. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol 2012; 23 (06) 1607-1616


 PDF
PDF  Views
Views  Share
Share

