Outcomes of pediatric glioblastoma treated with adjuvant chemoradiation with temozolomide and correlation with prognostic factors
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(02): 99-104
DOI: DOI: 10.4103/0971-5851.158838
Abstract
Background: Pediatric glioblastoma (pGBM) patients are underrepresented in major trials for this disease. We aimed to explore the outcome of pGBM patients treated with concurrent and adjuvant temozolomide (TMZ). Materials and Methods: 23 patients of pGBM treated from 2004 to 2010 were included in this retrospective analysis. Adjuvant therapy included conformal radiation 60 gray at 2 gray/fraction daily over 6 weeks with concurrent TMZ 75 mg/m 2 followed by six cycles of adjuvant TMZ 150-200 mg/m 2 (day 1-5) every 4 weeks. Kaplan-Meier estimates of overall survival (OS) were determined. Univariate analysis with log-rank test was used to determine the impact of prognostic variables on survival. Results: Median age at presentation was 11.5 years (range: 7-19 years) and M:F ratio was 15:8. All patients underwent maximal safe surgical resection; 13 gross total resection and 10 sub-total resection. At a median follow-up of 18 months (range: 2.1-126 months), the estimated median OS was 41.9 months. The estimated median OS for patients receiving only concurrent TMZ was 8 months while that for patients receiving concurrent and adjuvant TMZ was 41.9 months (P = 0.081). Estimated median OS for patients who did not complete six cycles of adjuvant TMZ was 9.5 months versus not reached for those who completed at least six cycles (P = 0.0005). Other prognostic factors did not correlate with survival. Conclusions: Our study shows the benefit of TMZ for pGBM patients. Both concurrent and adjuvant TMZ seem to be important for superior OS in this group of patients.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Pediatric glioblastoma (pGBM) patients are underrepresented in major trials for this disease. We aimed to explore the outcome of pGBM patients treated with concurrent and adjuvant temozolomide (TMZ).
Materials and Methods:
23 patients of pGBM treated from 2004 to 2010 were included in this retrospective analysis. Adjuvant therapy included conformal radiation 60 gray at 2 gray/fraction daily over 6 weeks with concurrent TMZ 75 mg/m2 followed by six cycles of adjuvant TMZ 150-200 mg/m2 (day 1-5) every 4 weeks. Kaplan-Meier estimates of overall survival (OS) were determined. Univariate analysis with log-rank test was used to determine the impact of prognostic variables on survival.
Results:
Median age at presentation was 11.5 years (range: 7-19 years) and M:F ratio was 15:8. All patients underwent maximal safe surgical resection; 13 gross total resection and 10 sub-total resection. At a median follow-up of 18 months (range: 2.1-126 months), the estimated median OS was 41.9 months. The estimated median OS for patients receiving only concurrent TMZ was 8 months while that for patients receiving concurrent and adjuvant TMZ was 41.9 months (P = 0.081). Estimated median OS for patients who did not complete six cycles of adjuvant TMZ was 9.5 months versus not reached for those who completed at least six cycles (P = 0.0005). Other prognostic factors did not correlate with survival.
Conclusions:
Our study shows the benefit of TMZ for pGBM patients. Both concurrent and adjuvant TMZ seem to be important for superior OS in this group of patients.
INTRODUCTION
Central nervous system (CNS) tumors are the second most common tumors of childhood, comprising 20% of all childhood malignancies, next only to leukemia. Only about 6% of all childhood CNS tumors are high-grade glioma, glioblastoma (GBM) being far less common than anaplastic astrocytoma, unlike in adults where GBM is the most common primary brain tumor[1] GBM connotes a dismal prognosis. The median age at diagnosis is 64 years, and this is primarily a disease of adulthood.[2] Treatment of GBM has evolved considerably over the past few years. Results of the landmark study by Stupp et al. established maximal safe surgery and radiation along with concurrent and adjuvant temozolomide (TMZ) as the standard of care for this disease.[3] Five years overall survival (OS) in this study was 9.8% versus 1.9% for the TMZ arm versus radiation alone arm. However, this landmark trial included patients in the age group of 18-70 and hence the extrapolation of benefit of this regimen in the pediatric population remains unknown. The encouraging results from the Stupp et al.[3] trial have been aptly supported by the molecular genetics of GBM. Promoter methylation of O6-methylguanine DNA methyltransferase (MGMT) has emerged as one of the strongest prognostic factors[4] for the outcome and benefit from TMZ. Few studies have evaluated the frequency and impact of MGMT methylation in pediatric GBM (pGBM) and it ranges from 11% to 60% in the reported literature and patients with methylated MGMT have superior survival as compared to their un-methylated counterparts.[4,5,6] Since pGBM is a rare entity; there are little data for the use of concurrent and adjuvant TMZ in these patients. Studies evaluating therapy and outcomes of pGBM separately are limited[7,8,9,10] and none of them have uniformly used TMZ. To the best of our knowledge, this retrospective case series is one of the largest of its kind evaluating a uniform schedule of chemo-radiation using TMZ in pGBM.
MATERIALS AND METHODS
We retrospectively analyzed institutional brain tumor database for cases of GBM (2004-2010). All patients with a diagnosis of GBM of age 20 years or less were selected for this study. Of a total of 270 cases of GBM treated during this period, 26 patients were found to be in the pediatric age group. On histopathology review, 3 patients were found to have other histology (2 were anaplastic astrocytoma and one was anaplastic oligoastrocytoma). Thus, 23 patients with confirmed histopathology of GBM (8.5% of total GBM) formed the study cohort for the present study. The study was approved by our institutional review board, and informed consent was obtained from all the patients (guardians of the patients in case of minors).
Patient-related factors including age, sex, symptoms, symptom duration and treatment-related factors viz. extent of surgical resection, histopathological findings, radiation treatment, concurrent and adjuvant chemotherapy, and toxicities during and after treatment were recorded from the medical record charts.
All patients underwent maximal safe resection (gross total excision [GTE] [>90% resection], sub-total excision [STE] [ < 90% resection] or decompression only) followed by concurrent chemo-radiation and adjuvant chemotherapy.
Radiation therapy
Postoperative radiation therapy was started within 4-6 weeks of surgery. Radiation therapy in all patients was planned by three-dimensional conformal techniques. Treatment volume was decided based on the preoperative magnetic resonance imaging (MRI) information. The initial clinical target volume (CTV) included the enhancing tumor and edema with 2 cm margin all around as seen in the preoperative T2-weighted/T2 fluid-attenuated inversion recovery MRI scan. The boost CTV encompassed the T1-weighted contrast enhancing tumor volume with an isotropic 2 cm expansion. A uniform expansion of 5 mm was given all around the CTV to generate the planning target volume (PTV). A dose of 50 gray in 25 fractions over 5 weeks was prescribed for the initial CTV followed by a boost of 10 gray in 5 fractions over 1-week to the boost CTV. The radiation was planned on Eclipse treatment planning system version 6.5 (Varian Medical Systems, Palo Alto, California, United States) and patients were treated on a linear accelerator, CL 2300 CD (Varian Medical System, Palo Alto, California, United States).
Chemotherapy
Concurrent TMZ was given at a dose of 75 mg/m2 daily, and adjuvant TMZ was started after a gap of 1-month. The first cycle was given at 150 mg/m2 (day 1-5) and depending on the tolerance increased to 200 mg/m2 in the next cycle for a minimum of six cycles every 4 weeks. Co-trimoxazole prophylaxis was not used.
Methylateion-specific polymerase chain reaction for O6-methylguanine DNA methyltransferase promoter
DNA methylation pattern of the MGMT gene promoter was determined by methylation-specific polymerase chain reaction (‘PCR). This procedure involves chemical modification of unmethylated, cytosine to uracil, followed by a nested, 2-Stage PCR. 500 ng of genomic DNA was extracted from formalin-fixed, paraffin-embedded tissue blocks using Ambion Recover All DNA extraction kit (Life Technologies, Carlsbad, CA, USA) according to manufacturer's protocol. Enzymatically methylated DNA was used as a positive methylation control, whereas normal lymphocytic DNA served as an unmethylation control.
Methylation-specific primers were used for PCR. First stage primer recognizes the bisulfite-modified template flanking the MGMT gene but does not discriminate between methylated and unmethylated alleles. Primer sequences of first PCR were 5⁚-GGATATGTTGGGATAGTT-3′ (forward primer) and 5′-CCAAAAACCCCAAACCC-3′ (reverse primer). PCR was carried out in a total volume of 25 μL with an initial denaturation step of 5 min at 94°C; followed by 40 cycles of 30 s at 94°C, 30 s at 52°C, and 30 s at 72°C and a final elongation step of 7 min at 72°C in DNA Engine Peltier Thermal Cycler (Bio-Rad, Hercules, California).
The Stage 1 PCR product was diluted 20-fold, and 2 μL of this dilution was subjected to a Stage 2 PCR. Methylation and unmethylation specific primers were used separately for each test. Primer sequences were as follows: 5′TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3′ (forward primer) and 5′AACTCCACACTCTTCCAAAAACAAAACA-3′ (reverse primer) for the unmethylated reaction (PCR product 83 base pair) and 5′-TTTCGACGTTCGTAGGTTTTCGC-3′ (forward primer) and 5′-GCACTCTTCCGAAAACGAAACG-3′ (reverse primer) for the methylated reaction (product size 91 base pair). The second PCR was performed with an initial denaturation step of 5 min at 94°C, followed by 35 cycles of 15 s at 94°C, 15 s at 62°C, and 15 s at 72°C and a final elongation step of 7 min at 72°C. Amplified products were separated on 4% agarose gel, ethidium bromide stained, and visualized under ultraviolet illumination.
Treatment monitoring and follow-up
Weekly complete blood counts were done during chemo-radiation and were repeated 1-2 days before each cycle of adjuvant chemotherapy. Patients were evaluated for toxicities using Common Terminology Criteria for Adverse Events version 2.0 (National Cancer Institute (National Institute of Health), Maryland, USA). Contrast-enhanced MRI of the brain was done after completion of the adjuvant treatment and subsequently every 3 months or earlier based on patient's symptomatology. Response evaluation was done by Macdonald's criteria.[11]
Statistical analysis
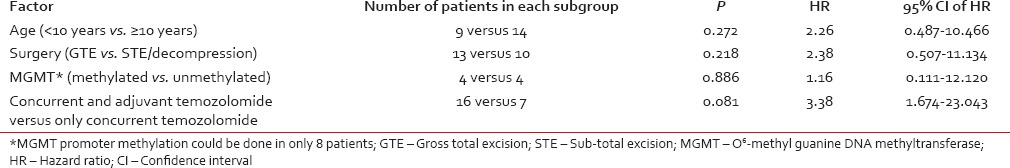
Survival outcomes were estimated from the time of diagnosis. OS was calculated from the time of diagnosis to the time of death from any cause. Kaplan-Meier method was used for survival analysis. Univariate analysis (log-rank test) was used to assess the impact of prognostic variables on survival. P < 0.05 was taken as significant, and SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The effect of the following prognostic variables on OS was evaluated: Concurrent versus concurrent and adjuvant TMZ, number of adjuvant chemotherapy cycles (6 cycles versus < 6 cycles), age (less or more than 10 years), extent of surgery and promoter methylation of MGMT.
RESULTS
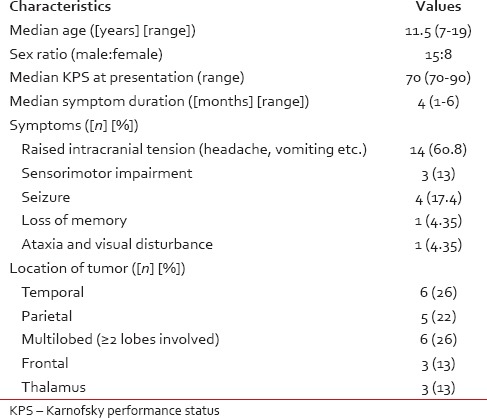
Of 23 patients, 15 were males and 8 were females. Most patients (60.8%) presented with features of raised intracranial tension. Rest of the patient characteristics are summarized in Table 1. 13 patients underwent a GTE, and 10 patients had STE of the tumor. There were no major morbidity/postoperative complications in any of the patients.
Table 1
Summarizes the patient characteristic
Tissue specimens of patients operated at other institution were reviewed by an expert neuropathologist. Tumors showed a high percentage of MIB-1 (labeling indexes, median being 28% [range: 10-45%]). p53 mutation status was available for 20 patients and was reported to be positive in 12 patients. of 8 patients, in whom sufficient biopsy material was available for testing promoter methylation of MGMT, 4 had methylated and 4 had unmethylated tumor.
All patients received a total radiation dose of 60 gray in 30 fractions over 6 weeks. Median interval between surgery and radiation therapy was 30 days (range: 15-35 days). All patients received concurrent TMZ during radiation. 7 patients received only concurrent TMZ while 16 patients received concurrent and adjuvant TMZ. The median number of adjuvant TMZ cycles was 6 (range: 2-12). Of the 16 patients, 11 patients received 6 cycles or more of adjuvant TMZ. One patient received adjuvant TMZ for 12 cycles under a protocol treatment.
Acute toxicities during radiation were mainly nausea, anorexia, headache, vomiting, and dermatitis. Grade 1 nausea was the most frequent accompaniment and seen in > 90% of the patients. 13 (56.5%) patients had Grade 2 nonhematological toxicities. 1 patients had Grade 2 (neutropenia), and 1 patient had Grade 3 (thrombocytopenia) during concurrent radiation. During adjuvant chemotherapy, 4 patients (17.3%) had Grade 3 thrombocytopenia, and 2 patients had Grade 3 neutropenia (8.7%).
Median follow-up duration for the entire cohort was 18 months (range: 2.1-126 months). At the time of last follow-up, 7 patients had expired and 11 were asymptomatic and free of disease. Estimated median OS of the entire cohort was 41.9 months. The 1-year and 2-year estimated OS was 69.6% and 60.9%, respectively [Figure 1]. Eight patients survived more than 41 months. Of these, one patient is surviving 126 months from the time of diagnosis. Three of the 4 patients with methylated MGMT and only 1 of 4 patients with unmethylated MGMT were alive at the time of last follow-up. MGMT methylation status was not known in the patient with the longest survival of 126 months.
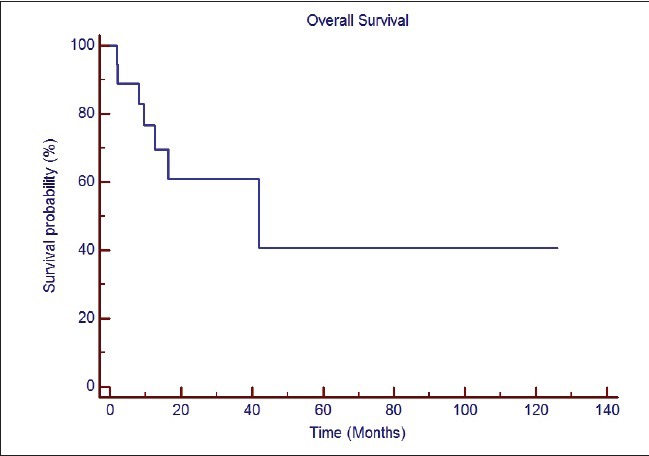
| Fig. 1 Overall survival of the entire cohort of patients
Adjuvant TMZ was associated with better survival as compared to no adjuvant TMZ (median survival 41.9 months vs. 8.06 months; P = 0.0812) [Figure 2]. At least 6 cycles of adjuvant TMZ were associated with significantly better survival as compared to < 6 cycles (median survival Not reached (NR) vs. 9.5 months; P = 0.0005). Rest of the prognostic variables did not impact survival significantly as summarized in Table 2. In view of small sample size and less number of events, multivariate analysis was not performed.
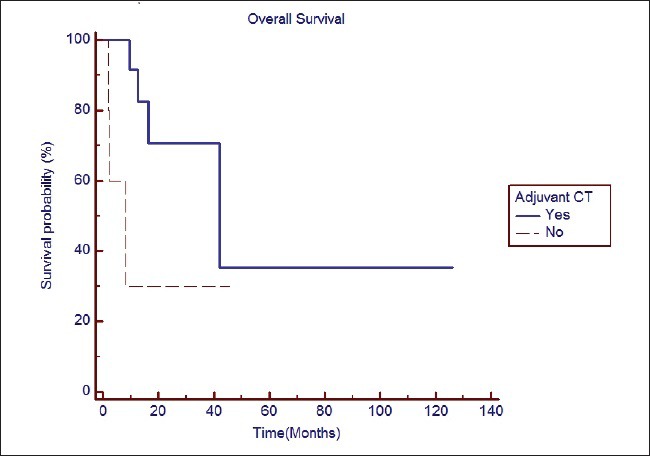
| Fig. 2 The impact of adjuvant chemotherapy with temozolomide on overall survival
Table 2
Impact of prognostic variables on survival (univariate analysis)
DISCUSSION
Most centers include surgery and adjuvant radiotherapy in the management of pGBM, but the role of chemotherapy is investigational. It originates from astrocytic lineage with a reported 3 years survival of under 20%. Central review of institutionally defined entities has shown discordance in diagnosis to the tune of 36%, with many being labeled as low-grade glioma upon central review,[12] raising further doubts as to the behavior and management outcome of this entity.
Despite having a denovo occurrence, pGBM exhibit high incidence of p53 mutations and low incidence of epidermal growth-factor receptor (EGFR) amplification,[5] which are features of adult secondary GBM. Loss of retinoblastoma protein expression is uncommon and p53 with bcl-2 over-expression is more often associated with ominous prognosis.[13] Suri et al.[14] have shown that p53 alteration is more frequent in pGBM to a tune of 63%, whereas EGFR over-expression was found in 23% with rare occurrence of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) deletion. In the present study, p53 mutation was seen in 60% of patients.
O6-methylguanine DNA methyltransferase promoter methylation in pGBM has been studied in only few case series[4,5,6] and the results are variable. In a study by Srivastava et al.,[6] MGMT promoter methylation was seen in 50% (10 out of 20) of the pGBM. In another study by Pollack et al.,[5] 85% of the pGBM patients (28 out of 33) had low expression of MGMT. In the study by Pollack et al.,[5] low levels of MGMT expression also correlated with improved progression-free survival (PFS). 5-year PFS of patients with low levels of MGMT was statistically superior as compared to those with increased levels of MGMT (29% vs. 0%; P = 0.013). In our study, 50% of the patients (4 out of 8) had promoter methylation of MGMT. However, this did not correlate with improved survival (P = 0.886).
The earliest reported large experience on pGBM was by Dohrmann et al.[15] in 1976. Nine of 43 patients in this study did not receive any therapy, and they concluded that hemispheric GBM presents at a later age and have a better outcome compared with brainstem GBM. Both dexamethasone therapy and postoperative radiotherapy appeared to improve survival. However, no concurrent or adjuvant chemotherapy was used in this study.
In a study on supratentorial pediatric malignant astrocytomas at St. Jude's Children Research Hospital,[16] 41 patients under 21 years were included, 25 of which were GBM. Chemotherapy was given to 33 patients, most common agents being cyclophosphamide, etoposide, vincristine, and cisplatin. OS and PFS at 3 years were 35% and 18%, respectively, with no difference between anaplastic astrocytoma and GBM. Extent of resection, even when controlled for tumor site appeared as the most important prognostic factor influencing outcome. We did not note any difference in survival based on the extent of surgical resection in our study. Partly, this could be due to patients undergoing surgery at another institution and inappropriate documentation of the extent of surgery by neurosurgeons. In our study, 40% of patients underwent surgery at a different institution.
Another study from Mexico[7] reporting on 16 patients reiterated the importance of the extent of resection, with a median survival following the sub-total resection at 21.48 months, compared to 33.80 months after GTE. This study did not give details of the type of adjuvant chemotherapy or number of patients receiving it. However, this study reports the longest survival in pGBM, mean survival being 54.97 months (range: 32.2-74.73 months). The survival reported in this study is in concordance with our study. The median OS in our study was 41.9 months, with eight patients surviving more than 41 months and one patient surviving 126 months. In yet another study by Song et al.,[10] 5-year OS for pGBM patients was found to be 40%. The results of these studies are pointers to a relatively longer OS in pGBM than their adult counterparts. A study by Das et al.[17] have shown younger age, higher MGMT promoter methylation and higher PTEN protein over-expression to be associated with long-term survival in GBM patients. However, the survival in our study could not be reliably associated with these molecular parameters, in view of the paucity of molecular findings from all patients.
Introduction of TMZ has revolutionized the management of adult GBM, it being the only drug with a clearly established survival benefit.[3] However, studies so far in pediatric brain tumors have failed to define its role in this patient population. A phase 2 study[18] in progressive high-grade or brainstem glioma showed only a 6-12% overall response rate with considerable toxicity in the form of prolonged myelosuppression resulting in treatment delays in 17% of cycles. Another study[19] on recurrent high-grade glioma (n = 20) administered 4-weekly TMZ 200 mg/m2 (day 1-5) and observed an overall response rate of 20% with median PFS of 2 months and median OS of 9 months. The authors recommended trials of TMZ as upfront or adjuvant therapy. Cohen et al.[20] evaluated the role of TMZ in the treatment of high-grade Glioma in children in a report from Children's Oncology Group (ACNS0126 study). 55 pGBM patients received concurrent chemo-radiotherapy with TMZ followed by adjuvant TMZ in this study and compared the results with Children's Cancer Group study (CCG-945). CCG-945 was a study which evaluated the role of adjuvant PCV (nitrosourea, vincristine, and prednisolone) for treatment of high-grade astrocytoma in children. TMZ failed to improve outcome in children with high-grade gliomas. The 3-year event-free survival rate for GBM was 7 ± 4% in ACNS0126 compared with 15 ± 5% in CCG-945 (P = 0.77). Though the ACNS0126 study did not demonstrate the superiority of TMZ over PCV regimen, there are several limitations to this comparison. This was not a head to head prospective comparison of the two treatment regimens. The toxicities of the two regimens were not compared, and this has important implication keeping in mind the overwhelming toxicity with PCV regimen. Finally, PCV regimen is now considered only in the management of recurrent high-grade gliomas and not in the adjuvant management of GBM. Hence, the clinical implication of this comparison is not helpful in the present scenario.
The results of our study reiterate the benefit of concurrent and adjuvant TMZ in the pediatric population. The OS being better when both concurrent and adjuvant TMZ is given (median OS 41.9 months vs. 8.06 months; P = 0.0812). The results of our study also stress the importance of completing 6 cycles of adjuvant TMZ. 6 cycles of adjuvant TMZ was associated with significantly better survival as compared to < 6 cycles (median survival NR vs. 9.5 months; P = 0.0005). The reason for some patients not receiving adjuvant TMZ was financial constraints rather than disease progression (except for 1 patient), thus implicating a true effect of benefit of adjuvant TMZ.
Our study was a retrospective analysis with its inherent shortcomings. Small sample size is a major limitation of our study; however, the rarity of this disease precludes reporting of large number of cases at a given point of time. Small sample size is also likely to impact statistical conclusions and results of our study should be taken as hypothesis generating rather than practice-changing. Absence of full molecular profile for all patients also did not allow us to get to a meaningful clinicopathological correlation. Despite all these, our study remains one of the largest till date to have uniformly explored the value of concurrent chemo-radiation in pGBM patients and would definitely be a valuable addition to existing literature.
CONCLUSION
Temozolomide is well-tolerated in the pediatric population with an acceptable toxicity profile. Adjuvant chemoradiation with TMZ yields impressive survival in children with GBM and it appears that both concurrent and adjuvant TMZ are important for obtaining superior survival outcomes for this otherwise dismal malignancy.
Footnotes
Source of Support: Nil
REFERENCES

| Fig. 1 Overall survival of the entire cohort of patients

| Fig. 2 The impact of adjuvant chemotherapy with temozolomide on overall survival


 PDF
PDF  Views
Views  Share
Share

