Outcomes, cost comparison, and patient satisfaction during long-term central venous access in cancer patients: Experience from a Tertiary Care Cancer Institute in South India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 232-238
DOI: DOI: 10.4103/0971-5851.195732
Abstract
Introduction: Prolonged treatment, frequent administration of chemotherapy, antibiotics and blood products in cancer patients requires long term venous access. Central venous catheters (CVC) inserted into the subclavian vein or internal jugular vein, peripherally inserted central venous catheters (PICC) and chemoport (CP) are the commonly used central venous access devices (CVAD). Aim: This study was envisaged to review our experience of CVADs over a one year period and analyze the outcome with regard to catheter life, reasons for removal, complications, patient satisfaction and cost comparison between the CVAD types in the Indian setting. Settings and Design: This was a prospective, observational study carried out in a tertiary care cancer institute. Materials and Methods: 180 CVADs placed in patients with hematological malignancies and solid tumors from January 2014 to December 2014 were included. Statistical Analysis Used: Data was analyzed using descriptive statistics, Mann Whitney U test. P < 0.05 was taken as statistically significant. Results: 180 CVADs were placed in 160 patients. The median catheter indwelling period was 76 days (16 days to 313 days) for CVC, 59 days (20days – 313 days) for PICC and 137 days (70 days – 258 days) for CP. 66 out of 160 patients developed complications (41.2%). 108 complication events were noted in 66 patients. There were 40 episodes of CRBSI. Out of the 68 mechanical complications, 37 were encountered during insertion of the CVAD and 31 were during the catheter indwelling period. Out of 160 patients, 138 (86.25%) were satisfied with the CVAD. The cost incurred for CVC/PICC (INR 4,480) was lower than that for CP (INR 24,150) and it was statistically significant (P < 0.0001). Our patients were highly satisfied with the CVAD. Conclusion: Use of CVC and PICC is a safe, reliable and cost saving way of administration of chemotherapy in developing countries. The incidence of complications and catheter loss was acceptable. Our patients were highly satisfied with the CVAD
Keywords
Central venous catheters - chemoport - complications - cost comparison - patient satisfaction - peripherally inserted central venous catheterPublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
Abstract
Introduction:
Prolonged treatment, frequent administration of chemotherapy, antibiotics and blood products in cancer patients requires long term venous access. Central venous catheters (CVC) inserted into the subclavian vein or internal jugular vein, peripherally inserted central venous catheters (PICC) and chemoport (CP) are the commonly used central venous access devices (CVAD).
Aim:
This study was envisaged to review our experience of CVADs over a one year period and analyze the outcome with regard to catheter life, reasons for removal, complications, patient satisfaction and cost comparison between the CVAD types in the Indian setting.
Settings and Design:
This was a prospective, observational study carried out in a tertiary care cancer institute.
Materials and Methods:
180 CVADs placed in patients with hematological malignancies and solid tumors from January 2014 to December 2014 were included.
Statistical Analysis Used:
Data was analyzed using descriptive statistics, Mann Whitney U test. P < 0.05 was taken as statistically significant.
Results:
180 CVADs were placed in 160 patients. The median catheter indwelling period was 76 days (16 days to 313 days) for CVC, 59 days (20days – 313 days) for PICC and 137 days (70 days – 258 days) for CP. 66 out of 160 patients developed complications (41.2%). 108 complication events were noted in 66 patients. There were 40 episodes of CRBSI. Out of the 68 mechanical complications, 37 were encountered during insertion of the CVAD and 31 were during the catheter indwelling period. Out of 160 patients, 138 (86.25%) were satisfied with the CVAD. The cost incurred for CVC/PICC (INR 4,480) was lower than that for CP (INR 24,150) and it was statistically significant (P < 0.0001). Our patients were highly satisfied with the CVAD.
Conclusion:
Use of CVC and PICC is a safe, reliable and cost saving way of administration of chemotherapy in developing countries. The incidence of complications and catheter loss was acceptable. Our patients were highly satisfied with the CVAD.
INTRODUCTION
Cancer patients require long-term venous access in view of prolonged treatment and frequent administration of chemotherapy, blood components, antibiotics, and total parenteral nutrition. Introduction of central venous catheters (CVCs) in the 1980s has revolutionized the care and quality of life of cancer patients.[1,2] These devices have also increased the patient satisfaction by minimizing the need for venipunctures and associated emotional trauma.
Central venous access devices (CVADs) include open-ended tunneled catheters, tunneled valve catheters, implantable subcutaneous chemoports (CPs), noncuffed nontunneled CVCs, and peripherally inserted central catheters (PICCs). The device is chosen based on the patient age, underlying disease, intensity, frequency and duration of treatment, type of solutions used and preference of the patient, and physician.[3] The cost of the device and its maintenance forms a crucial economic factor in the long-term management of cancer, especially in developing countries like India. It requires expert handling care and may be associated with complications.
Our aim was to review our experience of CVADs over a period of 1 year and to analyze the outcome regarding types of catheters used, difficulty during insertion, catheter indwelling period, incidence of infections and mechanical complications, and reasons for removal. In addition, we also attempted to compare costs involved in insertion and maintenance of CVADs in our institute and patient satisfaction.
MATERIALS AND METHODS
In this prospective, observational study, we studied the clinical profile of 160 patients with CVADs inserted in our institute from January 2014 to December 2014 after obtaining approval from the Institutional Ethical Committee. Patient age ranged between 16 and 63 years. One hundred and fifteen (71.88%) patients had hematological malignancies, and 45 (28.12%) had solid tumors. CVC was placed in the internal jugular or subclavian vein. PICC was placed in the cubital vein of the nondominant upper limb. CP was placed in the subclavian vein except one patient who underwent a femoral port placement owing to extensive venous thrombosis. CVADs were inserted by oncology residents using anatomical landmarks by the Seldinger technique. Chest radiograph was taken to confirm the position of the catheter before use. CVADs were used for the administration of chemotherapy and supportive care including antibiotics, blood products, and total parenteral nutrition. In this study, long-term central venous access was defined as catheter indwelling period of at least 2 weeks.
Protocol for catheter care
Our institute has a dedicated “Catheter Care Clinic.” When in use, CVCs were flushed using 2 cc of heparinized saline (100 U/ml) daily. When not in use, the CVCs were flushed twice weekly. CPs were accessed with a noncoring needle and flushed with 5 ml of heparinized saline. Exit site care and dressing change were done twice weekly. At each dressing change, exit site was examined for the presence of infection, catheter leak, retrograde flow, pyogenic discharge, evidence of venous thrombosis, and extravasation. Injection cap was changed every fortnight. A record of follow-up care of all patients was maintained. Patients were counseled about the importance of regular follow-up. One hundred and thirty-eight (86.25%) patients were compliant with the protocol of catheter care. Patients paying less than five visits to the clinic per month were considered noncompliant.
Catheter removal
Catheter removal was done under aseptic precautions. Local pressure at exit site was applied for 5 min, and sterile dressing applied after removal. The catheter was examined for breakage, obstruction, and thrombosis. The criteria for catheter removal included completion of proposed treatment regimen, death of the patient, development of catheter-related bloodstream infection (CRBSI) not responding to appropriate antibiotic and antifungal therapy, venous thrombosis, and persistent blockage.
Data regarding the catheter indwelling period cost incurred, complications encountered, and their management was collected and entered in a pretested unstructured pro forma.
Cost comparison
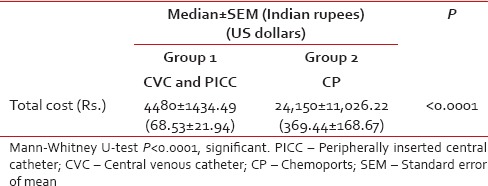
For each patient, total cost including the amount spent on insertion of device, maintenance, and management of complications, if any, was calculated. Tables Tables11 and and22 depict the details of the cost incurred at our institute. Cost estimates were calculated in Indian rupees (INRs) and US Dollars (USDs). The cost comparison was done between Group 1 (CVC and PICC) and Group 2 (CP). Cost data were compared using the Mann–Whitney U-test [Table 3].
Table 1
Cost of insertion and maintenance of central venous access device
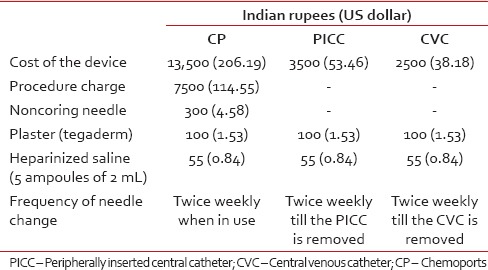
Table 2
Cost incurred during management of complications
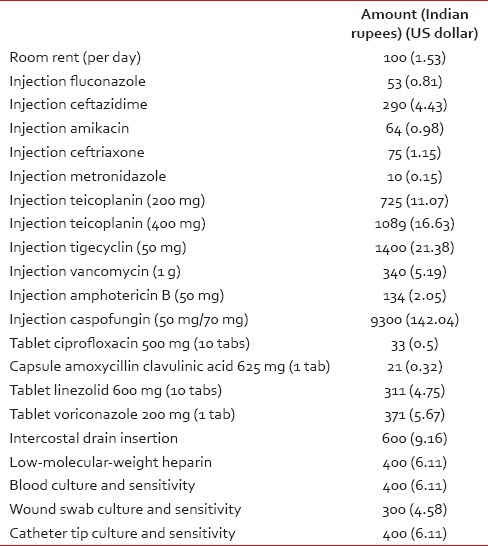
Table 3
Cost comparison
Patient satisfaction
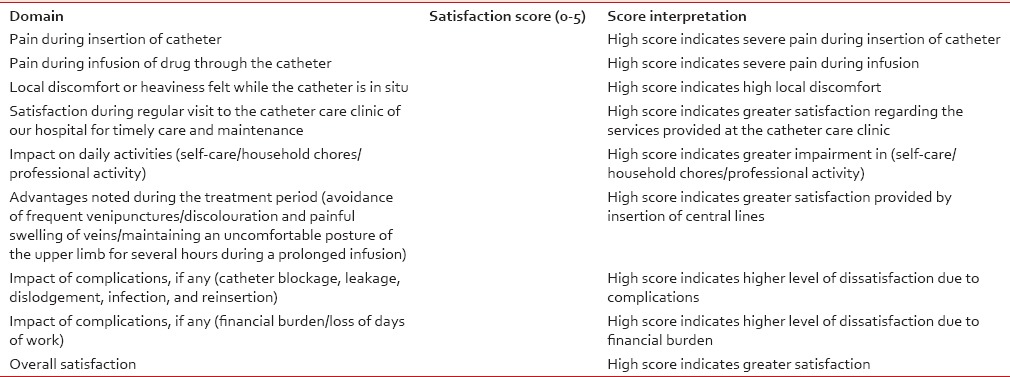
Patients were asked about their satisfaction with the CVAD initially after the 2 weeks of insertion and then during each hospital visit. A hospital devised unvalidated questionnaire to assess patient satisfaction was used based on the domains shown in Table 4.
Table 4
Questionnaire for assessment of patient satisfaction
Statistical analysis
Data were analyzed by SPSS 13 software using descriptive statistics and Mann–Whitney U-test for comparison of continuous variables across groups. P < 0.05 was taken as statistically significant.
RESULTS
There were 185 attempts at central venous access during our study period. Five were failures. Hence, 180 CVADs were placed in 160 patients. Twenty patients had more than one CVAD placement and each placement was counted as a new event for the analysis. One hundred and sixty-five (91.99%) were noncuffed, nontunneled, nonantibiotic impregnated CVC, four (2.22%) were PICC and 11 (6.11%) were CP.
Median catheter indwelling period
It was 76 days (interquartile range: 16–313 days) for CVC, 59 days (interquartile range: 20–313 days) for PICC, and 137 days (interquartile range: 70–258 days) for CP.
Cost comparison of central venous catheter and peripherally inserted central catheter versus chemoport
The average cost for both groups was determined as shown in Table 3. The cost incurred for CVC or PICC (INR: 4480) (USD: 68.53) was lower than that for CP (INR: 24150) (USD: 369.44), and it was statistically significant (P < 0.0001). Hence, CVC and PICC are a cost-saving option.
Patient satisfaction
Out of 160 patients, 138 (86.25%) were satisfied with the CVAD and 22 (13.75%) were not.
Complications
Table 5
Complication during insertion of central venous access devices
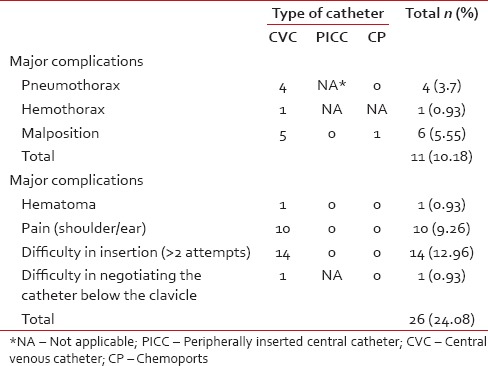
Table 6
Mechanical complications during catheter indwelling period
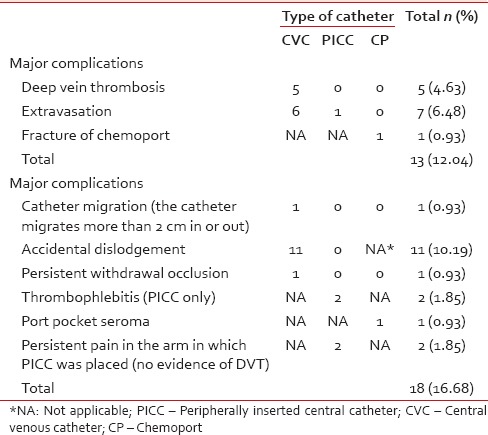
Major complications included pneumothorax, hemothorax, malposition, thrombosis, extravasation, fracture of CP, and CRBSI. Minor complications were defined as those that did not render the CVAD ineffective and were managed conservatively. The overall incidence of major mechanical complications was 22.2%, and CRBSI was 18.8%.
Out of forty episodes of CRBSI, 25 (62.5%) had exit site infections, and 21 (52.5%) had febrile neutropenia. We were able to salvage twenty CVADs (50%) with appropriate antibiotic therapy. There were only two deaths due to CRBSI in our study. Out of 123 blood cultures analyzed, 34 (27.6%) yielded isolates, predominantly Gram-positive. Staphylococcus aureus was the most common isolate overall (38.23%). Acinetobacter baumannii was the most common Gram-negative isolate (20.58%).
Reasons for removal of central venous access devices
Six months after the last entry in this series, 57 (31.66%) CVADs were still in use and 123 (68.33%) had been removed. CVAD was removed electively after the successful completion of therapy in 55 patients (30.5%) and after death in 19 patients (10.5%). Forty-nine (27.22%) were removed prematurely due to catheter-related complications such as CRBSI in twenty, extravasation in seven, DVT in five, and fracture of CP, hemothorax, catheter migration, persistent backache during infusion, catheter lumen occlusion with no backflow in spite of a gentle 5 mL heparinized saline flush, and persistent pain and swelling in the arm in 1 each. About 11 patients reported accidental dislodgement. One patient had a fracture of the CP. The fractured segment had embolized to the right ventricle. It was removed under angiographic guidance with a goose neck snare via femoral route through a venous sheath [Figures [Figures11 and and22].
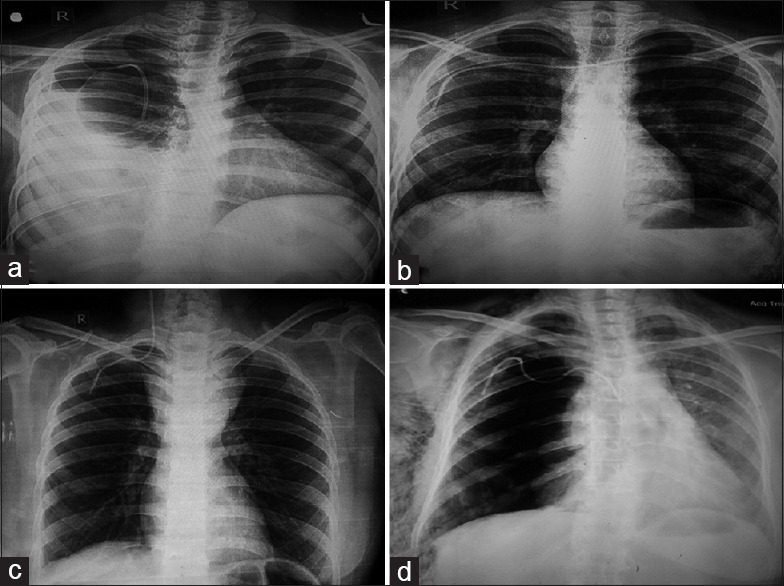
| Fig. 1 (a) Chest radiograph showing spontaneous catheter migration and right hemopneumothorax. Intercostal drain is in situ, (b) malposition: Catheter has crossed the midline, into the left subclavian vein, (c) malposition: Catheter is seen in the right internal jugular vein. Superior mediastinal mass is seen. Patient had T-lymphoblastic lymphoma, (d) right pneumothorax with extensive subcutaneous emphysema following insertion of right subclavian CVC
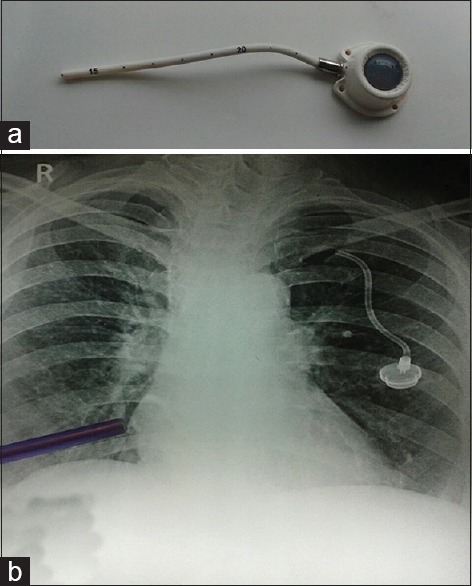
| Fig. 2 (a) Proximal segment of the fractured chemoport, (b) the blue pointer shows the distal segment of the fractured chemoport that had embolized to the right ventricle
Survival analysis of central venous access devices
A Kaplan–Meier survival analysis curve for time to catheter removal (all removals including treatment completion, death, and complications) by type of CVAD was plotted to visualize the survival rate over time [Graph 1]. The graph reveals the divergence of the survival curves between CVC/PICC and CP.
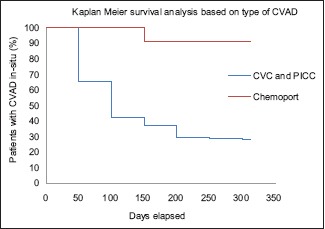
| Graph 1 Kaplan–Meier survival analysis curve for time to catheter removal (all removals including treatment completion, death, complication) by type of central venous access device
DISCUSSION
Central venous catheterization is a time-tested technique of quickly accessing the great veins. It scores over peripheral venous access regarding greater longevity, lower infection rates, multiple lumens for simultaneous administration of drugs, a route for administration of antibiotics, blood components, and central venous pressure monitoring.[4]
CVCs are classified by tip position, the material they are made up of or by the expected duration of use as short-term (days to weeks), intermediate-term (weeks to months), and long-term (months to years) access.
Gowardman et al.[5] and Norwood et al.[6] showed that the catheter colonization rate was least at the subclavian site among adults. We preferred the subclavian site for CVC insertion. Internal jugular vein was chosen in patients with a large mediastinal mass and those with breast cancer with extensive involvement of skin over the chest wall. We avoided the femoral vein for CVC placement due to increased risk of catheter colonization and thrombosis. We had one instance of femoral placement of CP as the patient had extensive DVT in the thorax.
The choice of the catheter is influenced by the expected duration of use, chemotherapy regimen, and patient preference.[7] We opted for PICC in those with AML-M3 due to the underlying coagulopathy. Patients underwent CVC placement during induction chemotherapy for acute leukemia and sepsis with difficult peripheral venous access as the procedure can be done with speed and ease. Patients receiving consolidation chemotherapy in acute leukemia and those with solid tumors were given the option of CVC or CP insertion. Most of our patients preferred CVC over CP due to affordability. Thus, the economic factor was an important reason which led to fewer insertions of CP in our study.
Median duration of catheter indwelling period was greatest for CP (137 days) and least for PICC (59 days). Jain et al. in their study, of 213 patients also reported similar findings with the catheter indwelling period being greatest in port patients (280 days) and least in PICC patients (59 days). Therefore, CP may be considered an effective tool in the long-term use of catheters in cancer patients.
The major limitation for the use of a CP in developing countries like India is the cost factor. This is evident by the low number of CPs in our study as compared with the CVC/PICC. Hence, we thought that a cost comparison analysis between the two groups would be justified to explain the preference for CVC/PICC in comparison with CP. We found that the average cost of insertion and maintenance of CP (INR: 24,150) was nearly six times that incurred for CVC/PICC (INR: 4480) and was statistically significant (P < 0.0001). This stark difference prohibited many of our patients from opting for the more desirable CP. Patel et al.[8] in their study of cost analyses between PICC and ports inserted for nonhematological malignancies in Australia, found that the cost of port insertion and removal was significantly higher than that of PICCs (US $3925.83 versus US $957.14) due to the requirement of surgical theaters. Similarly, in our study, the cost of CP insertion was significantly higher than that of CVC and PICC (Rs. 22000 vs. Rs. 2524). As per Patel et al.,[8] the cost of maintenance (US $9.22 vs. US $26.36 for ports vs. PICCs) and complications (US $1,567 vs. US $12,317.20 for ports vs. PICCs) associated with PICC lines is significantly higher than that for port devices. On the contrary, in our study, the cost of weekly maintenance (Rs. 855 vs. Rs. 255 for ports vs. PICC/CVC) and the average cost of complications (Rs. 13332 vs. Rs. 6082 for ports vs. CVC/PICC) was significantly higher for CP than CVC/PICC. This has probably resulted from the less number of patients in CP group. One patient in the CP group had CRBSI, which necessitated an expenditure of Rs. 107,476.
The majority of our patients (86.25%) were highly satisfied with their CVAD as compared to the cannulation of a peripheral vein. Patients were satisfied with the CVAD as frequent painful venipunctures could be avoided, it was not associated with discoloration of peripheral veins, and it alleviated the need to keep the upper limb in an inconvenient position during prolonged infusion. The cost incurred for maintenance of the CVAD and the possibility of a reinsertion in case of premature removal did not alter their replies. Some of those who had complications during catheter insertion or its indwelling period were not satisfied as it prolonged the hospital stay and made the experience unpleasant.
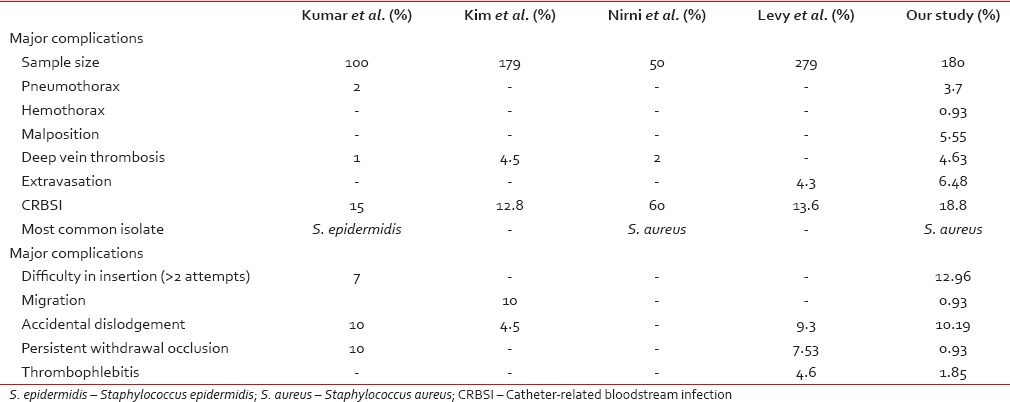
The overall incidence of catheter-related complications was 41.2% in our study with 22.4% mechanical and 18.8% infective. Jain et al.[3] had 19% overall complication incidence with 12% mechanical and 7% infective. Kim et al.[9] had 30.1% overall complication incidence with 18.3% mechanical and 12.8% infective. The higher incidence rate in our study can be explained by the inclusion of both major (such as occlusion, malposition, thrombosis, and infection) and minor complication factors (such as difficulty in insertion defined as more than two attempts, difficulty to negotiate the catheter below the clavicle, pain in shoulder/ear, persistent pain in the PICC placed arm, and extravasation) in our study, whereas the others included only major complications.
Among the complications encountered during the insertion of the catheter, difficulty in insertion (7.78%) was the most common. Likewise, difficulty in placement of catheters was found in 5% cases of Certofix® and 12.5% cases of Cavafix® by Kumar et al.[10] Four patients had pneumothorax (2.2%), and one had hemothorax (0.56%) during insertion of the catheter, which is similar to 2% pneumothorax reported by Kumar et al.[10] Primary malposition of CVAD was seen in five subclavian CVC and one CP. We inserted CVADs using landmark technique. Although ultrasound helps to guide the placement of CVAD, it has been shown to have no effect on the rate of complications or failures.[11] The malpositioned catheters were repositioned using a guidewire. When comparing the CP versus CVC/PICC during insertion of the catheter, we found only one case of malpositioned CP, whereas there were 36 complications in total in the CVC/PICC group. Although the CP was costlier, it had a lower incidence of complications during insertion.
Accidental dislodgement was the most common mechanical complication during the catheter indwelling period (10.19%). In our center, it is prevented by replacing the sutures whenever needed and education of patients and care givers. Extravasation was noted in 3.89%, deep vein thrombosis in 2.78%, and thrombophlebitis in 1.11% of cases, in our study. Kim et al.[9] reported an incidence of 4.5% thrombosis, whereas Nirni et al.[12] reported only 2% of thrombosis. The range in our study falls well within the ranges reported in the literature. Extravasation of drug and fluid was noted in 6.48%. Because of timely diagnosis, there were no reports of tissue necrosis. It may be prevented by regular aspiration and by infusing only isotonic crystalloids down the proximal lumen.[13] We found a very low incidence (0.56%) of persistent withdrawal occlusion, defined as inability to aspirate blood via catheter while the ability to infuse is being preserved, whereas Kumar et al.[10] reported incidence as high as 10% in Certofix® and 22.5% in Cavafix®. While comparing CP versus CVC/PICC group, CP group had only two complications (one seroma and one fracture) while CVC/PICC group had 29 during catheter indwelling period. Spontaneous fracture of CP varies from 0.4% to 1.8%.[14,15] The catheter fragment may lodge in the vena cava, right atrium, right ventricle, or the pulmonary artery.[16,17] We found one case with this rare complication.
The rates of CRBSI in different studies varied from as low as 7%[3] to as high as 60%.[9] In our study, it was 21.25%. In the study by Gorelick et al.,[18] the incidence of infection was 27% in those who were neutropenic at the time of catheter insertion. In our study, 18.5% of those whose absolute neutrophil count was below 500/mm3 during catheter insertion developed CRBSI. The rate of culture positivity in our study was 27.42% which was similar to that reported by Nirni (26%).[12] We found a slightly higher incidence of Gram-positive isolates (52.93%), which more than half were S. aureus. Kumar et al.[10] found Staphylococcus epidermidis, while Nirni.[12] found S. aureus as the most common isolate. Gram-negative organisms accounted for 47.05% of the isolates, with A. baumannii and Enterobacter aerogenes being the major isolates followed by 1 case each of Klebsiella pneumoniae, Citrobacter koseri, Pseudomonas aeruginosa, and Flavobacter species. Jain et al.[3] found the most common isolate to be P. aeruginosa. We managed to salvage 18 out of 31 CVC/PICC and two out of three CPs. However, 19 CVC/PICC and one CP had to be removed prematurely due to infection and there were two deaths related to CRBSI in CVC group.
A comparison of complications reported in various studies has been shown in Table 7.[9,10,11,19]
Table 7
Comparison of complication between the different studies
 The majority of catheters (72.77%) in our study were functioning at the time of catheter removal or are still in use. Treatment completion was the most common reason for catheter removal (30.5%) suggesting that most of the CVCs served the purpose they were meant for.
The majority of catheters (72.77%) in our study were functioning at the time of catheter removal or are still in use. Treatment completion was the most common reason for catheter removal (30.5%) suggesting that most of the CVCs served the purpose they were meant for.Our study shows that, in developing countries, CVC and PICC are safe, reliable, long lasting, and cost-saving options for long-term intravenous access in oncology patients as compared to CP. Until the cost of the CP reduces significantly, CVC/PICC will continue to play a major role in the management of cancer patients in developing countries.
Limitations
As there were fewer patients in the CP group as compared to CVC/PICC (due to the cost factor), we could not carry out the statistical analysis of comparison of various complications between the two groups.
A complete cost-effectiveness analysis was not carried out within this study due to the lack of a validated quality of life questionnaire related to CVC placement (covering functional status, sleep, bathing and hygiene disturbance, patient costs, time taken off work for CVAD insertion, and hospital attendances), which could be used to measure quality-adjusted life years as an outcome as was suggested by Patel et al.[8]
Financial support and sponsorship
Nil.
Conflicts of interest
REFERENCES

| Fig. 1 (a) Chest radiograph showing spontaneous catheter migration and right hemopneumothorax. Intercostal drain is in situ, (b) malposition: Catheter has crossed the midline, into the left subclavian vein, (c) malposition: Catheter is seen in the right internal jugular vein. Superior mediastinal mass is seen. Patient had T-lymphoblastic lymphoma, (d) right pneumothorax with extensive subcutaneous emphysema following insertion of right subclavian CVC

| Fig. 2 (a) Proximal segment of the fractured chemoport, (b) the blue pointer shows the distal segment of the fractured chemoport that had embolized to the right ventricle


 PDF
PDF  Views
Views  Share
Share

