Octamer 4 expression and lymph node metastasis in ductal carcinoma of breast: Are they associated?
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2019; 40(01): 63-66
DOI: DOI: 10.4103/ijmpo.ijmpo_154_18
Abstract
Purpose: Octamer 4 (Oct-4) is a transcription factor which is required for the self-renewal and pluripotency of embryonic stem cells and germ cells. In this study, we tried to examine the association of expression of Oct-4 with lymph node metastasis in ductal carcinoma of the breast. Methods: The study was conducted on a total of 45 cases of invasive ductal carcinoma of breast, no special type. Oct-4 expression was studied on paraffin-embedded sections by immunohistochemistry. Results: Oct-4 expression was seen in 22.2% of cases. No statistically significant association was found between the expression of Oct-4 and histological type, tumor size, histological grade, and lymph node metastasis. Of Oct-4 positive tumor, 80% of cases showed lymph node metastasis, as compared to 62.85% without Oct-4 expression. However, the association was statistically insignificant. Conclusion: Oct-4 expression can be a promising biomarker of carcinogenesis, metastatic potential, and prognosis of carcinoma breast. However, the study with larger sample size is needed to establish the clinicopathological potential of this biomarker.
Publication History
Article published online:
08 June 2021
© 2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Purpose: Octamer 4 (Oct-4) is a transcription factor which is required for the self-renewal and pluripotency of embryonic stem cells and germ cells. In this study, we tried to examine the association of expression of Oct-4 with lymph node metastasis in ductal carcinoma of the breast. Methods: The study was conducted on a total of 45 cases of invasive ductal carcinoma of breast, no special type. Oct-4 expression was studied on paraffin-embedded sections by immunohistochemistry. Results: Oct-4 expression was seen in 22.2% of cases. No statistically significant association was found between the expression of Oct-4 and histological type, tumor size, histological grade, and lymph node metastasis. Of Oct-4 positive tumor, 80% of cases showed lymph node metastasis, as compared to 62.85% without Oct-4 expression. However, the association was statistically insignificant. Conclusion: Oct-4 expression can be a promising biomarker of carcinogenesis, metastatic potential, and prognosis of carcinoma breast. However, the study with larger sample size is needed to establish the clinicopathological potential of this biomarker.
Introduction
The most common type of breast cancer is ductal carcinoma, and about 80% are of invasive ductal type. Invasive ductal carcinoma affects most commonly elderly women.[1] The most important prognostic factor of breast carcinoma is lymph node metastasis.[2],[3]
Octamer 4 (Oct-4) is a transcription factor containing POU DNA-binding domain.[4] Oct-4 expressions are associated with self-renewal of undifferentiated embryonic stem cells.[5] Oct-4 has been regarded as a biomarker of adult stem cells, and it is highly expressed in liver, mammary, and gastric stem cells.[6] Currently, the expression of Oct-4 has been found in embryonal carcinoma, germ cell tumor, testicular carcinoma in situ, seminoma, and dysgerminoma.[7],[8],[9]
The aim of this study was to examine the Oct-4 expression in invasive ductal carcinoma breast and to correlate Oct-4 expression with tumor size, histological tumor grade, and regional lymph node metastases.
Methods
The consecutive study was carried out on the formalin-fixed paraffin-embedded tissue sections of 45 consecutive patients with breast cancer including 30 cases with lymph node metastasis and 15 cases without lymph node metastasis. Patients with infiltrating ductal carcinoma breast, who underwent modified radical mastectomy, were included in the study, while patients with invasive carcinoma of breast other than no special type and male breast ductal carcinoma were excluded from the study.
For all specimens, 4-μm thick sections of paraffin-embedded tissue were prepared on poly-L-lysin-coated slides and subjected to Oct-4 immunohistochemical staining. The sections were deparaffinized in xylene and rehydrated through graded alcohol. For microwave, antigen retrieval citrate buffer of pH 6 was used. The slides were incubated overnight with Oct-4 primary antibody (clone: EP143, rabbit monoclonal antibody, BioSB, USA), followed by incubation with the superenhancer for 30 min and then with secondary antibody for 20 min. DAB was added for 10 min. Next, counterstaining in hematoxylin was carried out followed by dehydration, clearing, and mounting. Tissue sections from seminoma were included in each run as a positive control for Oct-4.
Statistical analysis
Associations between categorical variables (side of breast involved, tumor size, tumor grade, lymph node status, estrogen receptor expression, progesterone receptor expression, Her2 expression, and molecular subtypes) were analyzed using the Chi-square test. Two-sided P < 0>
Results
Patient characteristic
A total of 45 patients were enrolled in this study. The mean (±standard deviation) age was 48.15 (±11.35) years. Out of 45 cases, 66.7% lesions were left sided. The largest diameter of tumor in most (68.9%) cases was 2–5 cm. Invasive ductal carcinoma cases were graded according to the Nottingham modification of Bloom–Richardson grading;[10] out of 45 cases, 44.4% of cases were categorized as Grade II.
Immunohistochemical Results
Positive expression of ER was seen in 24.4% of cases, and positive PR expression was present in 24.4% of cases. Her2neu positivity was noted in 44.4% of cases. On molecular subtyping, 44.4% of cases were basal like, 31.1% were Her2+ type, 13.3% were luminal B type, and 11.1% of cases were luminal-A type.
Expression of Oct-4 immunostain was studied in all cases of infiltrating ductal carcinoma, no special type. About 22.2% of cases showed nuclear and/or cytoplasmic positivity in ≥1% tumor cells and were considered positive for Oct-4 [Figure 1] and [Figure 2]. About 77.8% of cases were either negative for Oct-4 expression or showed nuclear and/or cytoplasmic staining in <1>
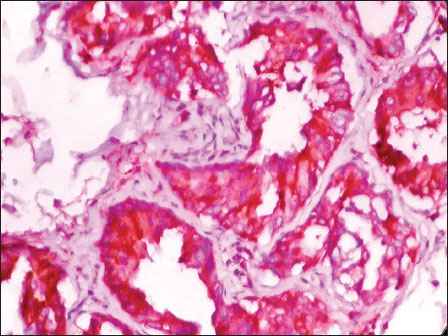
| Figure 1: Photomicrograph of invasive breast carcinoma, no special type showing cytoplasmic positivity with Octamer 4 (DAB, ×400)
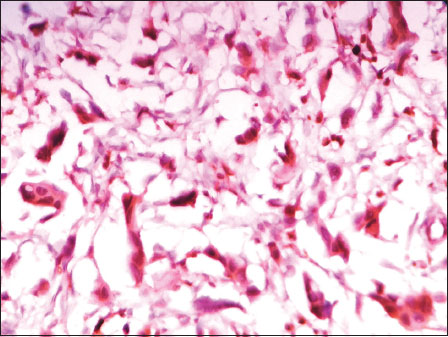
| Figure 2: Photomicrograph of invasive breast carcinoma, no special type showing nuclear positivity with Octamer 4 (DAB, ×400)
Correlation between Octamer 4 expression and clinicopathological features
There was no statistically significant association of Oct-4 expression with side involved by tumor (P = 0.482), largest tumor diameter (P = 0.371), histological grade (P = 0.151), ER [removed]P = 0.194), PR [removed]P = 0.643), Her2 [removed]P = 0.108), and molecular subtype (P = 0.353). Of Oct-4 positive tumor, 80% of cases showed lymph node metastasis, as compared to 62.85% without Oct-4 expression. However, the difference was not statistically significant (P = 0.310). The details are summarized in [Table 1].
|
Variables |
Total cases |
Number of cases (%) |
P* |
|
|---|---|---|---|---|
|
Oct-4 positive |
Oct-4 negative |
|||
|
*Chi-square test. ER – Estrogen-receptor; PR – Progesterone receptor; Her2 – Human epidermal growth factor receptor 2; Oct-4 – Octamer 4 |
||||
|
Side of breast |
||||
|
Right |
14 |
5 (35.7) |
9 (64.28) |
0.482 |
|
Left |
25 |
5 (20.0) |
25 (80.0) |
|
|
Bilateral |
1 |
0 (0.00) |
1 (100) |
|
|
Tumor size (largest dimension) (cm) |
||||
|
<2> |
6 |
0 (0.00) |
6 (100.0) |
0.371 |
|
2-5 |
31 |
8(25.8) |
23 (75.19) |
|
|
>5 |
8 |
2 (25.0) |
6 (75.0) |
|
|
Lymph node status |
||||
|
Positive |
30 |
8 (26.6) |
22 (73.3) |
0.310 |
|
Negative |
15 |
2 (13.3) |
13 (86.6) |
|
|
Histological grade |
||||
|
Grade I |
7 |
3 (42.8) |
4 (57.1) |
0.151 |
|
Grade II |
20 |
2 (10.0) |
18 (90.0) |
|
|
Grade III |
18 |
5 (27.7) |
13 (72.2) |
|
|
ER status |
||||
|
Positive |
11 |
4 (36.3) |
7 (63.6) |
0.194 |
|
Negative |
34 |
6 (17.6) |
28 (82.3) |
|
|
PR status |
||||
|
Positive |
11 |
3 (27.2) |
8 (72.7) |
0.643 |
|
Negative |
34 |
7(20.5) |
27 (79.4) |
|
|
Her2 status |
||||
|
Positive |
20 |
5 (25.0) |
15 (75.0) |
0.108 |
|
Negative |
22 |
3 (13.6) |
19 (86.3) |
|
|
Equivocal |
3 |
2 (66.6) |
1 (33.3) |
|
|
Molecular subtype |
||||
|
Luminal A |
5 |
1 (20.0) |
4 (80.0) |
0.353 |
|
Luminal B |
6 |
3 (50.0) |
3 (50.0) |
|
|
Her2+ |
14 |
2 (14.28) |
12 (85.71) |
|
|
Basal like |
20 |
4 (20.0) |
16 (80.0) |
|
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90
- Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB. et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983; 52: 1551-7
- Saez RA, McGuire WL, Clark GM. Prognostic factors in breast cancer. Semin Surg Onco 1989; 5: 102-10
- Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 2005; 6: 872-84
- Monsef N, Soller M, Isaksson M, Abrahamsson PA, Panagopoulos I. The expression of pluripotency marker Oct 3/4 in prostate cancer and benign prostate hyperplasia. Prostate 2009; 69: 909-16
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: Evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 2005; 26: 495-502
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000; 24: 327-6
- Chavez L, Bais A, Vingron M, Lehrach H, Adjaye J, Herwig R. In silico identification of a core regulatory network of OCT4 in human embryonic stem cells using an integrated approach. BMC Genomics 2009; 10: 314
- van de Geijn GJ, Hersmus R, Looijenga LH. Recent developments in testicular germ cell tumor research. Birth Defects Res C Embryo Today 2009; 87: 96-113
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M. et al.Cancer Incidence in Five Continents. IARC Scientific Publication No-160. Vol. 9. Lyon: IARC; 2007.
- Liu CG, Lu Y, Wang BB, Zhang YJ, Zhang RS, Lu Y. et al. Clinical implications of stem cell gene Oct-4 expression in breast cancer. Ann Surg 2011; 253: 1165-71
- Cai S, Geng S, Jin F, Liu J, Qu C, Chen B. et al. POU5F1/Oct-4 expression in breast cancer tissue is significantly associated with non-sentinel lymph node metastasis. BMC Cancer 2016; 16: 175
- Gwak JM, Kim M, Kim HJ, Jang MH, Park SY. et al. Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget 2017; 8: 36305-18
- Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei X. et al. Oct-4 and nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget 2014; 5: 10803-15
- Abou Gabal HH, Abu-Zeid RM, El-Maraghy MN. Implication of Oct-4 in breast carcinoma from initiation to lymph node metastasis: An Immunohistochemical study. Egypt J Pathol 2016; 36: 194-200
- Hassiotou F, Hepworth AR, Beltran AS, Mathews MM, Stuebe AM, Hartmann PE. et al. Expression of the pluripotency transcription factor OCT4 in the normal and aberrant mammary gland. Front Oncol 2013; 3: 79
- Madjd Z, Hashemi F, Shayanfar N, Farahani E, Zarnani AH, Sharifi AM. et al. OCT-4, an embryonic stem cell marker expressed in breast, brain and thyroid carcinomas compared to testicular carcinoma. Iran J Cancer Prev 2009; 2: 167-73
Address for correspondence
Publication History
Article published online:
08 June 2021
© 2019. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Photomicrograph of invasive breast carcinoma, no special type showing cytoplasmic positivity with Octamer 4 (DAB, ×400)

| Figure 2: Photomicrograph of invasive breast carcinoma, no special type showing nuclear positivity with Octamer 4 (DAB, ×400)
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90
- Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB. et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983; 52: 1551-7
- Saez RA, McGuire WL, Clark GM. Prognostic factors in breast cancer. Semin Surg Onco 1989; 5: 102-10
- Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 2005; 6: 872-84
- Monsef N, Soller M, Isaksson M, Abrahamsson PA, Panagopoulos I. The expression of pluripotency marker Oct 3/4 in prostate cancer and benign prostate hyperplasia. Prostate 2009; 69: 909-16
- Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: Evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 2005; 26: 495-502
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000; 24: 327-6
- Chavez L, Bais A, Vingron M, Lehrach H, Adjaye J, Herwig R. In silico identification of a core regulatory network of OCT4 in human embryonic stem cells using an integrated approach. BMC Genomics 2009; 10: 314
- van de Geijn GJ, Hersmus R, Looijenga LH. Recent developments in testicular germ cell tumor research. Birth Defects Res C Embryo Today 2009; 87: 96-113
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M. et al.Cancer Incidence in Five Continents. IARC Scientific Publication No-160. Vol. 9. Lyon: IARC; 2007.
- Liu CG, Lu Y, Wang BB, Zhang YJ, Zhang RS, Lu Y. et al. Clinical implications of stem cell gene Oct-4 expression in breast cancer. Ann Surg 2011; 253: 1165-71
- Cai S, Geng S, Jin F, Liu J, Qu C, Chen B. et al. POU5F1/Oct-4 expression in breast cancer tissue is significantly associated with non-sentinel lymph node metastasis. BMC Cancer 2016; 16: 175
- Gwak JM, Kim M, Kim HJ, Jang MH, Park SY. et al. Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget 2017; 8: 36305-18
- Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei X. et al. Oct-4 and nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget 2014; 5: 10803-15
- Abou Gabal HH, Abu-Zeid RM, El-Maraghy MN. Implication of Oct-4 in breast carcinoma from initiation to lymph node metastasis: An Immunohistochemical study. Egypt J Pathol 2016; 36: 194-200
- Hassiotou F, Hepworth AR, Beltran AS, Mathews MM, Stuebe AM, Hartmann PE. et al. Expression of the pluripotency transcription factor OCT4 in the normal and aberrant mammary gland. Front Oncol 2013; 3: 79
- Madjd Z, Hashemi F, Shayanfar N, Farahani E, Zarnani AH, Sharifi AM. et al. OCT-4, an embryonic stem cell marker expressed in breast, brain and thyroid carcinomas compared to testicular carcinoma. Iran J Cancer Prev 2009; 2: 167-73


 PDF
PDF  Views
Views  Share
Share

