Non-Hodgkins lymphoma with lactic acidosis at presentation: A case report of a rare oncologic emergency
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(01): 83-85
DOI: DOI: 10.4103/0971-5851.133728
Abstract
Lactic acidosis (LA) has been reported to be associated with high grade lymphoma as a terminal event. Its causes are multi-factorial. It can either occur due to overproduction of lactic acid by rapidly dividing tumor or due to its underutilization due to involvement of liver by lymphomatous deposits. The prognosis of lymphoma associated with LA is dismal. We present a patient of non-Hodgkins lymphoma (NHL) who presented with LA, after an initial response succumbed.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Lactic acidosis (LA) has been reported to be associated with high grade lymphoma as a terminal event. Its causes are multi-factorial. It can either occur due to overproduction of lactic acid by rapidly dividing tumor or due to its underutilization due to involvement of liver by lymphomatous deposits. The prognosis of lymphoma associated with LA is dismal. We present a patient of non-Hodgkins lymphoma (NHL) who presented with LA, after an initial response succumbed.
INTRODUCTION
Lactic acidosis (LA) is known to occur in patients of leukemia and lymphoma as a terminal event. It occurs in rapidly proliferating tumors. It was first described in patients with acute leukemia by Field et al. in 1963.[1] Diagnostic criteria of LA are pH less than 7.35 along with plasma lactate concentration greater than 5 mmol/l.[2] It is reported to occurs in adults with leukemia and lymphoma. The presence of LA portends poor prognosis. However, LA as the initial presentation of non-Hodgkins lymphoma (NHL) is extremely rare.
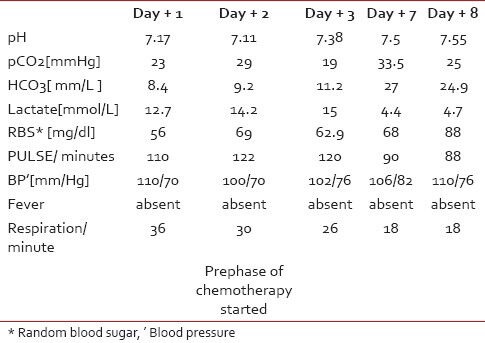
A 35-year-old male presented with intermittent fever, loss of appetite and fatigue of 5 months duration. He had weight loss of nearly 25 kg during this period. On examination, his ECOG performance status was 4. He was afebrile with a pulse of 140/min, and a respiratory rate of 36/minute. Pallor, pedal edema along with right cervical lymphadenopathy was present. On systemic examination a firm mass of 10 cm was felt in central abdomen. On evaluation, hemoglobin was 5.1 gm/dl, WBC 9200/cu mm, platelet count 2,72,000/cu mm; renal and liver functions were normal with serum (sr) urea 37 mg/dl, sr creatnine 0.7 mg/dl, sr bilirubin 0.5 mg/dl, sr SGOT 52 units/L, sr SGPT 27 units/L and sr alkaline phosphatase 260 units/L. His sr LDH was raised with a value of 876 IU/L and sr albumin was markedly low 1.8 gm/dl. His viral markers were negative. Blood gas analysis showed pH 7.17, pCO2 23 mmHg, HCO3 8.4mmol/L, serum lactate 12.7 mmol/L, suggestive of metabolic acidosis [Table 1]. Computerized tomography (CT) scan showed dilated small bowel loops with mural thickening along with the presence of homogenous mesenteric and retroperitoneal masses measuring about 7 cm along with mediastinal lymph nodes. A biopsy of the abdominal lymph node revealed NHL (subtype DLBCL), immunohistochemistry of tumor was positive for CD20 and negative for CD3 and CD10. Bone marrow was not involved by abnormal lymphoid cells. Hence, the final stage was 3BE with an IPI score of 3/5. As blood sugar, urea and creatinine were normal, blood cultures were sterile, no history of intake of metformin or other medication causing LA and other common causes of metabolic acidosis were ruled out. The patient was started on soda bicarbonate, broad spectrum antibiotics, although no focus of infection was detected. Simultaneously, he was given cyclophospamide 500 mg on day 1 and day 2, dexamethasone 8 mg three times daily day 1 to day 4 and doxorubicin 30 mg on day 3. Patient's vitals improved gradually and after 1 week, his lactate level came down along with improvement in pH and bicarbonate levels. He subsequently received six courses of R-CHOP (rituximab, cyclophospamide, doxorubicin, vincristine, prednisolone)-based chemotherapy. He had a partial response that was short lasting and developed progression of lymphoma within 2 months of sixth course of R-CHOP. He subsequently died of progressive disease. LA recurred during the latter part of his progressive disease.
Table 1
Showing following parameters [arterial blood gas, blood sugar, vitals] before and after adding chemotherapy
|
DISCUSSION
Lactic acid is a degradation product of glucose in anaerobic conditions. In anaerobic conditions, pyruvate is converted to lactate. There are two types of LA, hypoxic (type A) and non-hypoxic (type B). Malignancy is often associated with type B. Causes of LA in malignancy are either due to overproduction of LA or under utilization of LA or both. Tissue hypoxia is one of the causes of overproduction of LA. Tumor cell have a different metabolic milieu as compared to non-malignant cells. Rapidly dividing tumors outgrow their blood supply; this creates relative hypoxia within the tumor bed. This leads to anaerobic glycolysis with activation of LDH and production of lactate.[3] However, hypoxia is not the only mechanism of high lactate level in malignancy. Otto Warburg in 1930 demonstrated that cancer cells rely on glycolysis even in the presence of oxygen, a phenomenon known as aerobic glycolysis “Warburg effect”.[4] Apart from hypoxic environment, elevated lactate level may also occur due to liver metastasis, over expression of type II hexokinase, regulatory effect of insulin-like growth factor. In malignant cells even under aerobic conditions there is increased activation of mitochondrial-bound type 2 hexokinase, a rate-limiting enzyme of glycolysis. This occurs due to aberrant production of insulin-like growth factors along with its binding proteins and tumor necrosis factor (TNF-α) by tumor tissue.[5] In our patient, liver was not involved by lymphoma. The poor prognostic impact of LA in malignancy can be judged from fact that out of the total 29 published cases of lymphoma with LA, 25 eventually died (Pubmed search database). Hence, LA in lymphoma portends a very poor prognosis. The molecular basis for this may be due to a functional impairment of cytotoxic T cells. Activated T lymphocytes are also dependent on glycolysis during the period of proliferation and cytokine production and thus produce lactate.[6] Lactate molecule must be transported outside the T lymphocyte for uninterrupted glycolysis. This transport requires proton-linked monocarboxylate transporters, which co-transport protons and lactate anions following a concentration gradient.[7] But lactate produced by the tumors disrupts the lactate gradient between intracellular and extracellular compartment of cytotoxic T cells present within the tumor bed. This creates functional impairment of the T lymphocytes in tumor micro milieu. Treatment of underlying malignancy is essential in managing LA.[8] Role of buffering agents like soda bicarbonate to treat LA is only supportive and temporary.[9] Anemia can rarely be a cause of LA in this case as LA persisted even after blood transfusion. In this case though there were other confounding factors like low albumin, B-symptoms and bulky disease that are causes of poor prognosis so we can say that LA has an additive effect for poor prognosis in high-risk cases of lymphoma. The literature review of the cases of LA in lymphoma also suggests that these cases were having one or other high-risk features along with LA.[5,10]
CONCLUSION
LA is rare in patients with lymphoma. It is associated with high tumor burden along with aggressive biological and clinical behavior and portends a poor prognosis in spite of early management of treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Field M, Block JB, Rall DP. Lactic acidosis in acute leukemia. Clin Res 1963;11:193-7.
- Luft D, Deichsel G, Schulling RM, Stein W, Eggstein M. Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol 1983;80:484-9.
- Secomb TW, Hsu R, Dewhirst MW, Klitzman B, Gross JF. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys1983;25:481-9.
- Warburg O. [On the facultative anaerobiosis of cancer cells and its use in chemotherapy]. Munch Med Wochenschr 1961;103:2504-6.
- Sillos EM, Shenep JL, Burghen GA, Pui CH, Behm FG, Sandlund JT. Lactic acidosis: A metabolic complication of hematologic malignancies: Case report and review of the literature. Cancer 2001;92:2237-46.
- Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8 effector T cells. J Immunol 2005;174:4670-7.
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol 1993;264:C761-82.
- Chan FH, Carl D, Lyckholm LJ. Severe lactic acidosis in a patient with B-cell lymphoma: A case report and review of the literature. Case Rep Med 2009;2009:534561.
- Graf H, Leach W, Arieff AI. Metabolic effects of sodium bicarbonate in hypoxic lactic acidosis in dogs. Am J Physiol 1985;249:630-5.
- Ruiz JP, Singh AK, Hart P. Type B Lactic Acidosis Secondary to Malignancy: Case Report, Review of Published Cases, Insights into Pathogenesis, and Prospects for Therapy. ScientificWorldJournal 2011;11:1316-24.
References
- Field M, Block JB, Rall DP. Lactic acidosis in acute leukemia. Clin Res 1963;11:193-7.
- Luft D, Deichsel G, Schulling RM, Stein W, Eggstein M. Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol 1983;80:484-9.
- Secomb TW, Hsu R, Dewhirst MW, Klitzman B, Gross JF. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys1983;25:481-9.
- Warburg O. [On the facultative anaerobiosis of cancer cells and its use in chemotherapy]. Munch Med Wochenschr 1961;103:2504-6.
- Sillos EM, Shenep JL, Burghen GA, Pui CH, Behm FG, Sandlund JT. Lactic acidosis: A metabolic complication of hematologic malignancies: Case report and review of the literature. Cancer 2001;92:2237-46.
- Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8 effector T cells. J Immunol 2005;174:4670-7.
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol 1993;264:C761-82.
- Chan FH, Carl D, Lyckholm LJ. Severe lactic acidosis in a patient with B-cell lymphoma: A case report and review of the literature. Case Rep Med 2009;2009:534561.
- Graf H, Leach W, Arieff AI. Metabolic effects of sodium bicarbonate in hypoxic lactic acidosis in dogs. Am J Physiol 1985;249:630-5.
- Ruiz JP, Singh AK, Hart P. Type B Lactic Acidosis Secondary to Malignancy: Case Report, Review of Published Cases, Insights into Pathogenesis, and Prospects for Therapy. ScientificWorldJournal 2011;11:1316-24.


 PDF
PDF  Views
Views  Share
Share

